Smc5/6 is an evolutionarily conserved octameric complex composed of 2 Structural Maintenance of Chromosomes (SMC) subunits (Smc5 and Smc6) and 6 non-SMC elements (Nse1-6).Citation1 Similarly to its sister SMC complexes, cohesin and condensin, Smc5/6 is thought to embrace DNA strands within its ring-shaped structure. Cohesin and condensin are required for the establishment of cohesion between sister chromatids and for chromatin compaction, respectively. Smc5/6 contributes to topological processes and recombinational repair,Citation1 but its essential roles in chromosome metabolism remained elusive.
In a recent study conducted in the lab, we set out to investigate the functions of Smc5/6 in unperturbed conditions.Citation2 For this purpose, we first separated them in defined time windows, by establishing cell cycle regulated-alleles that enable restriction of essential Saccharomyces cerevisiae Smc5/6 components to either S or G2/M, using so-called S- and G2- tags. Previously reported distinct chromatin binding patterns for Smc5/6 in S and G2/M and the sensitivity of mutants to replication stress reagents Citation3,4 suggested crucial roles of this complex in S phase. However, diverging from this prediction, the essential functions of Smc5/6 segregated fully and selectively to G2/M, indicating that Smc5/6 can well provide for chromosome integrity functions if supplied after the bulk chromosome replication Citation2 ().
Figure 1. Schematic representation of Smc5/6 essential functions manifested in G2/M. Smc5/6 cooperates with Sgs1-Top3-Rmi1 in resolving recombination intermediates arising during endogenous DNA damage tolerance, and with the Rrm3 helicase in facilitating replication through vulnerable natural pausing site elements.
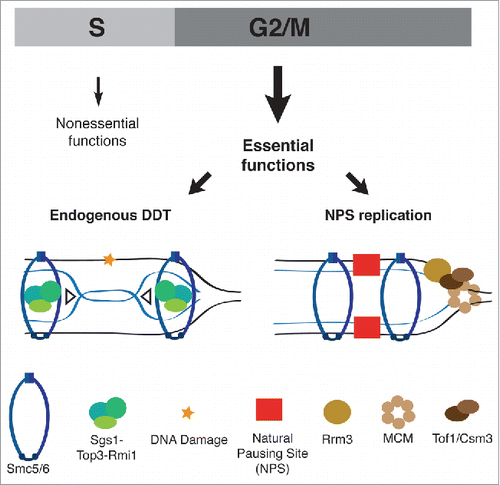
Conversely, limiting Smc5/6 components to S phase had adverse effects on viability, commensurate with the efficiency and timeliness of the S-tagged proteins degradation.Citation2 For instance, successful restriction of SMC5 and NSE4 expression to S phase resulted in lethality, and limitation of NSE1 and NSE2 to S phase caused severe slow growth. Interestingly, we uncovered that one of the S phase-restricted smc5/6 alleles generated in our study, S-SMC6, was hypomorphic. That is, it was proficient for S phase functions, but its levels dropped in G2/M. The small amounts of S-Smc6 proteins present in G2/M were sufficient to support cell viability, but further interference with Smc5/6 function caused lethality. This unique allele made possible genetic screens that allowed identification, for the first time, of DNA metabolism pathways that rely specifically on the G2/M function of Smc5/6. Two classes of mutants stood out from our screen. They related to the metabolism of DNA damage tolerance (DDT) recombination structures induced by endogenous replication stress and replication through natural pausing sites (). These findings provided us the genetic framework to investigate the roles of Smc5/6 in the above-mentioned processes, which we did using combinatorial approaches.
For the first identified process, our results revealed that Smc5/6 is crucial to cooperate with Top3 and the RecQ helicase Sgs1/BLM, mutated in cancer-prone Bloom syndrome patients, in resolving recombination intermediates induced by endogenous replication stress (). This phenomenon resembled the one previously identified in damaging conditions, where both Sgs1-Top3 and Smc5/6 complexes contribute to timely resolution of DDT recombination intermediates induced by replication-associated damage.Citation4,5 Interestingly, we found that the lethality of cells concomitantly mutated in Sgs1-Top3 and Smc5/6 was dependent on the conserved PCNA polyubiquitylation pathway, so far primarily characterized in response to DDT induced by exogenous DNA damage.Citation5 Moreover, cells with low postreplicative levels of Smc5/6 relied for viability on alternative G2/M-restricted resolution pathways mediated by structure-specific endonucleases that can introduce crossovers and cause genome rearrangements.Citation6 These findings provide new handles to investigate endogenous replication stress and its consequence for proliferation and genome integrity.
For the second process, our screen uncovered Smc5/6 to be required for viability in cells mutated in the DNA helicase Rrm3, known to facilitate replication through natural pausing sites (NPS). Notably, we found Smc5/6 itself to be enriched at NPS genome-wide and to co-localize with Rrm3 on chromatin. Previous work indicated that NPS are the equivalent of mammalian common fragile sites and represent at-risk genomic elements.Citation7 However, very little is known about how these regions are being maintained and on the DNA transactions responsible for their fragility. Importantly, in our recent work, we found that Smc5/6 functionality is essential to prevent chromosome fragility and accumulation of X-shaped recombination structures at those vulnerable regions.Citation2 Moreover, specifically at pausing sites, the late-appearing recombination intermediates – detected in smc5/6 mutants but not in wild-type cells – depended on the conserved replication fork pausing complex, Tof1-Csm3 (Tim-Tipin in mammalian cells). Tof1-Csm3 was also responsible for the lethality of G2/M function-defective smc5/6 hypomorphic mutants additionally mutated in the DNA helicase Rrm3. Thus, Smc5/6 is essential for limiting pausing- and Tof1-Csm3-dependent toxic recombination events that cause genome alterations and chromosome fragility at NPS (). These findings are crucial for understanding the etiology of the genome rearrangements associated with specific at-risk genomic loci and bring insights in the molecular basis of their fragility.
In conclusion, our findings indicate Smc5/6 as a keystone regulator of genome integrity via its general roles in directing DDT recombination intermediate resolution genome-wide, as well as unique roles in modulating recombination at topologically constrained regions, such as those happening during prolonged pausing (). The results thus shed light on Smc5/6 essential roles during proliferation, and provide frameworks for future investigations of chromosome metabolism processes that are important for development and genome integrity.
References
- Jeppsson K, et al. Nat Rev Mol Cell Biol 2014; 15:601-14; PMID: 25145851; http://dx.doi.org/10.1038/nrm3857
- Menolfi D, et al. Mol Cell 2015; 60:835–846; PMID:26698660; http://dx.doi.org/10.1016/j.molcel.2015.10.023
- Lindroos HB, et al. Mol Cell 2006; 22:755-67; PMID:16793545; http://dx.doi.org/10.1016/j.molcel.2006.05.014
- Branzei D, et al. Cell 2006; 127:509-22; PMID:17081974; http://dx.doi.org/10.1016/j.cell.2006.08.050
- Branzei D, et al. Nature 2008; 456:915-20; PMID:19092928; http://dx.doi.org/10.1038/nature07587
- Szakal B, et al. EMBO J 2013; 32:1155-67; PMID:23531881; http://dx.doi.org/10.1038/emboj.2013.67
- Song W, et al. Proc Natl Acad Sci U S A 2014; 111:E2210-8; PMID:24799712; http://dx.doi.org/10.1073/pnas.1406847111
