The regulation of cell-cycle phase transition is dependent upon the interactions of 3 classes of proteins; cyclin-dependent kinases (Cdks), cyclins and Cdk inhibitors such as the Cip/Kip family member p27Kip1. In the canonical model, cyclin binding to Cdks forms an activated Cdk complex leading to substrate phosphorylation and cycle progression, which is disrupted by additional binding of Cdk inhibitors. Moreover, it has been demonstrated that a novel family of cyclin-like proteins, which share no amino acid sequence homology with classical cyclins, also bind to Cdks and promote proliferation. Speedy A (SpyA) is one such cyclin-like protein.Citation1
The recent paper by Al Sorkhy et al., 2016Citation2 provides insight into novel regulation of cell cycle progression. The trimeric complex of Speedy A (cyclin-like protein), p27Kip1 (Cdk inhibitor) and Cdk2 autonomously regulates both Cdk2 activity and p27 stability, albeit in a differential manner (). This finding raises two important points; firstly, what are the potential functions of this complex and secondly how is the formation of this complex regulated in vivo?
Figure 1. A schematic overview of known Speedy A/p27/Cdk2 interactions. The possible heterodimeric and –trimeric interactions of the Speedy A/p27/Cdk2 triangle. These complex interaction may explain how Speedy A promotes proliferation in different contexts.
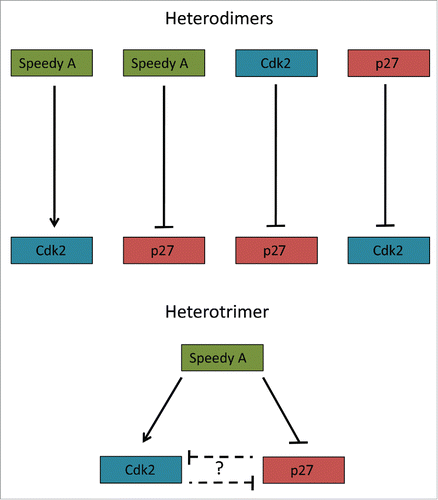
A clue to the first point can be found in the gene expression analysis which shows that Speedy A is principally expressed in the testes. Implication of Speedy A in meiosis is not new.Citation3 However, these findings suggest that Speedy A may act as a compensatory regulator of Cdk2/p27 in meiosis if traditional cyclin regulation of Cdk2 fails. Furthermore, unlike Speedy B genes, Speedy A displays remarkable sequence similarity across species.Citation1 Given that Cdk2 is essential for proper chromosomal pairing during meiosis it would make sense to evolve stringent regulatory controls of Cdk2 in meiotic tissues.Citation4
Additionally, it is known that Speedy A more readily associates with p27 than Cdk2 and that Cdk2 facilitates Speedy A binding to p27. We can now add to that picture with the finding that direct binding of Speedy A to p27 leads to p27 destabilization whereas direct binding of Speedy A to Cdk2 has no indirect effect on p27 stability. Given the plethora of literature showing that Cdk2 destabilises p27 through phosphorylation at T187 (for a review see Citation5), it is not clear why these data show that direct binding of Speedy A to p27 or Cdk2 in complex shows differential effects on p27 stability. Perhaps the conformation of the complex serves a dual purpose, whereby the primary function is to facilitate both Speedy A-mediated p27 degradation and Cdk2 activation and the secondary function is to protect p27 from excessive degradation by Cdk2 while spatially localizing p27 and Cdk2 to facilitate subsequent p27-mediated inhibition of Cdk2 activity. Conversely, p27 may function solely as an assembly factor in the same way it has been implicated as an assembly factor for Cdk4/cyclin D/p27 complexes.Citation6 In this model, p27 would facilitate Speedy A binding to Cdk2 and upon p27 degradation the complex would dissociate and Cdk2 would revert to its inactivate state.
Understanding the regulation of trimeric complex interactions is more difficult, however protein stoichiometry is likely important. Since Speedy A binds to Cdk2 and p27 independently, as does Cdk2 to Speedy A and p27, we can envision 4 different complexes: Cdk2/Speedy A, Cdk2/p27, Speedy A/p27, and the heterotrimeric Cdk2/Speedy A/p27 complex. Assuming that the concentration of Cdk2 and p27 are constant, we can now theoretically increase the Speedy A concentration and hypothesize the effect on the different complexes. At low Speedy A concentrations, most likely only the heterodimeric complexes (Cdk2/Speedy A and Speedy A/p27) would form. Once Speedy A is increased to an optimal concentration, the heterotrimeric complex would predominate. At very high concentrations (relative to Cdk2 and p27), it is possible that less of the heterotrimeric complex is formed and the formation of the dimeric complexes is favored. Similar observations were made for the formation of multimeric complexes in several biological systems.Citation7 This, in combination with the observed effect of Speedy A on p27 degradation, would result in highly dynamic complex behavior and could explain some of the confusing results obtained in the past.
The Speedy/RINGO family of cyclin-like proteins is emerging as an important alternative regulator of Cdks, however many questions remain unanswered. The discovery of a novel Speedy A/p27/Cdk2 triangle begins to unmask the mechanisms regulating Speedy A activation of Cdk2, yet more functional analysis of this complex is needed if we are to understand its significance in vivo.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Chauhan S, et al.. Cell Mol Life Sci 2012; 69:3835-50; PMID:22763696; http://dx.doi.org/10.1007/s00018-012-1050-1
- Al Sorkhy M, et al. Cell Cycle 2016; 15(1): 127–135; PMID: 26771716; http://dx.doi.org/10.1080/15384101.2015.1121327
- Gutierrez GJ, et al. Nat Cell Biol 2006; 8:1084-94; PMID:16964245; http://dx.doi.org/10.1038/ncb1472
- Viera A, et al. J Cell Sci 2009; 122:2149-59; PMID:19494131; http://dx.doi.org/10.1242/jcs.046706
- Kaldis P. Cell 2007; 128:241-4; PMID:17254963; http://dx.doi.org/10.1016/j.cell.2007.01.006
- LaBaer J, et al. Genes Dev 1997; 11:847-62; PMID:9106657; http://dx.doi.org/10.1101/gad.11.7.847
- Bray D, Lay S. Proc Natl Acad Sci USA 1997; 94:13493-8; PMID:9391053; http://dx.doi.org/10.1073/pnas.94.25.13493
