The Rab family of proteins are key small GTPases that play essential roles in membrane trafficking. The Rab proteins cycle between an inactive GDP-bound state and an active GTP-bound state. This GDP/GTP cycle is regulated by 2 key molecules. The first is a group of guanine nucleotide exchange factors (GEF), which is able to switch Rab from a GDP-bound form to a GTP-bound form, allowing it to recruit specific effectors to regulate various membrane trafficking processes. The second molecule is a GTPase-activating protein (GAP), which facilitates the GTPase activity of Rab proteins.
Rab11 is involved in various biological processes, including endocytic recycling, exocytosis, cell elongation, cell migration, and cell division, by controlling intracellular membrane trafficking. Rab11 is also related to various diseases, such as neurodegenerative diseases, diabetes, and cancers. Although multiple Rab11 GAPs and effectors have been identified, our knowledge about Rab11 GEF is still limited.
Recently, we identified REI-1 (Rab Eleven Interacting protein 1) as a new Rab11 GEF functioning in Caenorhabditis elegans (C. elegans) embryos.Citation1 In the C. elegans germline, RAB-11.1 (Rab11 in C. elegans) plays multiple roles during oocyte-to-embryo transition by dynamically changing its localization.Citation2 In growing oocytes, RAB-11.1 localizes to the recycling endosomes and late-Golgi, and regulates yolk receptor recycling. RAB-11.1 transiently changes its location to cortical granules immediately before fertilization and regulates their exocytosis after fertilization. RAB-11.1 then re-distributes to late-Golgi and recycling endosomes, and contributes to cytokinesis during embryonic cell division. To identify novel upstream regulators for RAB-11.1, we conducted a yeast two-hybrid screening using a GDP-fixed RAB-11.1 mutant as bait, and identified REI-1. REI-1 has a SH3-binding protein 5 (SH3BP5) domain, but lacks a Vps9 or DENN domain, which is found in other known Rab GEF proteins. We found another REI-1 homolog on the C. elegans genome and named it REI-2. REI-2 has a F-BAR domain in addition to the SH3BP5 domain. The REI family proteins are conserved in metazoans, including the fly and human (). REI-1 specifically binds to a GDP-bound form and a nucleotide-free form of RAB-11.1, but not to a GTP-bound form, in a similar manner to the known small GTPase GEF. Interestingly, REI-1 shows strong GEF activity for RAB-11.1 in vitro in the presence of liposomes, but not in their absence. A human REI-1 homolog, SH3BP5 (also called Sab; SH3 domain-binding protein, which preferentially associates with Bruton's tyrosine kinase) exhibits GEF activity for human Rab11 in the absence of liposomes, which is also strongly facilitated by liposome addition. REI-2 and SH3BP5/Sab contain a F-BAR domain and a BAR domain, respectively, both of which are known to form dimers and bind to membranes. REI-1 also has long helical structures similar to these domains, and directly binds to liposomes in vitro, suggesting its membrane binding ability. These observations suggest that the GEF activity of the REI family proteins is facilitated by membrane binding. Interestingly, REI-1 also shows GEF activity for human Rab11, suggesting that the Rab11 GEF activity is well conserved across species.
Figure 1. (Top) Domain structures of the REI/SH3BP5 family proteins. (Bottom) A working model of REI-1 function in C. elegans embryos. REI-1 recruits RAB-11.1 to late-Golgi by exchanging bound GDP for GTP. Activated RAB-11.1 is then distributed to the cleavage furrow via post-Golgi vesicles for proper cytokinesis.
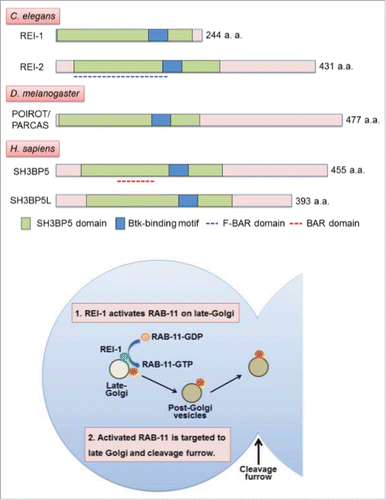
REI-1 localizes to late-Golgi and cortical granules in growing oocytes. After fertilization, REI-1 mainly localizes to late-Golgi, and partially overlaps with RAB-11.1 localization. Loss of REI-1 caused a cytokinesis delay of early stage embryos but did not affect yolk receptor recycling and cortical granule exocytosis, suggesting a specific function for REI-1 during the developmental stage. Although RAB-11.1 accumulates at the cleavage furrow and functions during cytokinesis, REI-1 and the late-Golgi marker SYN-16 are not recruited to the cleavage furrow. These results suggest that RAB-11.1 is activated by REI-1 on late-Golgi and subsequently targeted to the cleavage furrow via post-Golgi vesicles for proper cytokinesis in C. elegans embryos ().
In Drosophila oocytes, Rab11 and a REI-1 homolog (POIROT/PARCAS) are involved in the posterior localization of oskar mRNA and OSKAR proteins, respectively, and are required for germ cell proliferation and abdomen development.Citation3,4 Although the relationship between Rab11 and POIROT/PARCAS has not been studied, POIROT/PARCAS may activate Rab11 as a GEF and regulate the polarized transport or localization of oskar mRNA/OSKAR proteins in Drosophila oocytes. A human REI-1 homolog, SH3BP5/Sab, was originally identified as a Bruton's tyrosine kinase (Btk)-binding protein.Citation5 Interestingly, the overexpression of SH3BP5/Sab in B-cells inhibited the auto- and trans-phosphorylation activity of Btk and various B cell antigen receptor-mediated events, suggesting that SH3BP5/Sab negatively regulates Btk-related cytoplasmic signaling in B cells. SH3BP5/Sab is also reported to be phosphorylated by c-Jun N-terminal kinase (JNK).Citation6 In mammals, SH3BP5/Sab may have a distinct function in regulating Btk- and JNK-mediated signal transduction pathways or indirectly affect it by controlling Rab11 activity. There is another human homolog, SH3BP5L, but it has not been extensively studied. In contrast, C. elegans has no apparent Btk/Tec homologs in its genome. In addition, JNK binding and phosphorylated sites are not conserved in REI-1 and REI-2. Thus, REI-1 and REI-2 may exhibit the GEF activity independent of such kinases in C. elegans.
Our study reveals a novel type of Rab11 GEF family, which is well conserved across organisms (from worm to human). Although the loss of RAB-11.1 causes severe embryonic lethality in C. elegans, the rei-1; rei-2 knockout mutant exhibits a relatively mild phenotype, such as delayed cytokinesis and reduced progeny size, suggesting the existence of other Rab11 GEFs. A homolog of CRAG, which is a DENN protein (a homolog of human DENND4A, DENND4B, DENND4C) required for Rhodopsin trafficking in Drosophila,Citation7 is one of the candidates for an alternative Rab11 GEF. Since Rab11 is a multifunctional small GTPase, its function would be regulated by distinct types of GEFs at precise positions and timing. Further studies of the REI/SH3BP5 family proteins would not only reveal novel GEF-mediated mechanisms for regulating Rab11 functions in animal development, but would also provide new perspectives for connecting membrane trafficking with cell signaling.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Sakaguchi A, et al. Dev Cell 2015; 35:211–21; PMID: 26506309; http://dx.doi:10.1016/j.devcel.2015.09.013
- Sato M, et al. J Cell Sci 2008; 121:3177–86; PMID: 18765566; http://dx.doi: 10.1242/jcs.034678
- Dollar G, et al. Development 2002; 129:517–26; PMID: 11807042
- Sinka R, et al. Development 2002; 129:3469–78; PMID: 12091316
- Matsushita M, et al. Biochem Biophys Res Commun 1998; 245:337–43; PMID: 9571151
- Wiltshire C, et al. Biochem J 2002; 367:577–85; PMID: 12167088
- Xiong B, et al. PLoS Biol 2012; 10:e1001438; PMID: 23226104; http://dx.doi: 10.1371/journal.pbio.1001438
