ABSTRACT
The Schizosaccharomyces pombe MBF complex activates the transcription of genes required for DNA synthesis and S phase. The MBF complex contains several proteins, including the core components Cdc10, Res1 and Res2, the co-repressor proteins Yox1 and Nrm1 and the co-activator Rep2. It has recently been shown how MBF is regulated when either the DNA damage or the DNA synthesis checkpoints are activated. However, how MBF is regulated in a normal unperturbed cell cycle is still not well understood. We have set up a genome-wide genomic screen searching for global regulators of MBF. We have crossed our knock-out collection library with a reporter strain that allows the measurement of MBF activity in live cells by flow cytometry. We confirm previously known regulators of MBF and show that COP9/signalosome and tRNA methyltransferases also regulate MBF activity.
Introduction
During its life cycle, fission yeast cells face with a critical decision between vegetative growth, sexual conjugation, or remaining in stationary phase. This decision point, known as Start in yeasts and restriction point in metazoans, occurs in late G1 phase of the cell cycle. Genetic analyses have identified several proteins that are required for passage through Start in fission yeast. Among them, we can find the cyclin-dependent kinase, Cdc2 Citation1 and the activity of the transcription factor MBF, which is a multimeric complex whose core proteins are Cdc10, Res1 and Res2.Citation2-4 MBF, which is the functional analog of mammalian pRB/E2F, drives the G1-to-S wave of transcription, controlling the expression of some genes that are directly or indirectly required for DNA synthesis, such as cdc18 (the fission yeast homolog to CDC6), cdt1 and the gene coding for ribonucleotide reductase, cdc22.Citation3,5,6
We have shown that while cig2 is one of the genes that is under the control of MBF, the protein encoded by this gene, the cyclin Cig2, is also part of a negative feed-back loop that phosphorylates and inhibits MBF.Citation7 Another level of regulation is achieved by the repressor system Nrm1/Yox1. It has been shown that Yox1 binds the MBF complex through the co-repressor Nrm1, mainly at the end of S phase and during G2, when MBF-dependent transcription is down-regulated.Citation8-11 However, when DNA replication is challenged (i.e., treatment with hydroxyurea), Yox1 is phosphorylated by the effector kinase of the DNA synthesis checkpoint, Cds1. Once phosphorylated, Yox1 is released from MBF and MBF-dependent transcription is activated until cells overcome the block to DNA replication. Cells harboring a Yox1 mutant (Yox1-SATA) that cannot be phosphorylated by Cds1 are unable to induce MBF-dependent transcription when DNA replication is challenged with hydroxyurea. Interestingly, Yox1-SATA cells are still able to induce MBF-dependent transcription during S phase of a normal unperturbed cell cycle, pointing to the fact that Yox1 phosphorylation has a role in the DNA replication checkpoint, but not during a normal unperturbed S phase. Contrary to this positive effect on MBF-dependent transcription by the DNA synthesis checkpoint, in response to DNA damage (i.e., γ-irradiation, MMS treatment, etc) the outcome has been shown to be the opposite: MBF-dependent transcription is inhibited. This is achieved by direct phosphorylation of 2 specific residues located at the C-terminal domain of Cdc10 by the effector kinase of the DNA-damage checkpoint, Chk1.Citation12 This phosphorylation induces the release of MBF from its target promoters, repressing MBF-dependent transcription. When these residues in Cdc10 are mutated to non-phosphorylatable amino acids, fission yeast cells are highly sensitive to DNA damaging agents. Thus, Yox1 and Cdc10 couple normal cell cycle regulation and the DNA-synthesis and DNA-damage checkpoints in a single transcriptional complex.
Like its mammalian counterpart, the regulated activity of the MBF complex is essential for the normal G1-to-S transition: cells with hypoactive MBF complex are unable to complete S phase while cells with hyperactive MBF show genomic instability.Citation8 Thus, tight and precise regulation of the MBF complex activity is essential to avoid major problems, no matter whether in a unicellular cell or in a pluricellular organism. In this work, we describe a genome-wide genetic screening to identify all non-essential fission yeast proteins required for regulation of the MBF complex activity. This screening identified known regulators of MBF activity (like Res1, Res2, Yox1, Nrm1 and Rep2), but also some proteins of the COP9/signalosome and several tRNA methyltransferases.
Results
Cdc22-YFP as a reporter strain to measure MBF activity in vivo
We wanted to carry out a genome-wide screening of S. pombe to identify potential MBF regulators. The first step was to select an appropriate reporter with enough sensitivity to allow measurement of its activity directly on cells in culture, but also with good reproducibility between different biological replicates or conditions. We generated different strains that either were expressing chimeras of a fluorescent protein (YFP or GFP) fused to MBF-regulated genes (Cdc22-YFP, Yox1-GFP or Tos4-GFP) or were expressing YFP directly under the control of MBF-regulated promoters (pcdc18, pcdt2, pmik1 or ptos4).Citation3,11,13,14 By microscopic observation, we could detect fluorescence in each of the 7 strains that we tested, but with different intensity. Since we wanted to establish a method that would allow quantifying this fluorescence, we decided to determine their intrinsic fluorescence on the cytometer. As shown in , only the strain expressing Cdc22-YFP in its own locus showed differential fluorescence (10-fold increase fluorescence) when compared with the parental wild type strain (compare WT with Cdc22-YFP bars). Next, since we also needed a control reporter to measure constitutive transcription, we placed a cassette containing mRFP under the control of housekeeping promoters with constitutive cell cycle transcription, such as srp2, act1 or sty1 (). Red fluorescence was detected in all 3 strains and quite proportional to the strength of the promoter, with sty1 promoter being the strongest one.
Figure 1. Cdc22-YFP detects changes in MBF activity. (A) Quantification of the yellow fluorescence by FACS of the strains indicated on the right. Inset: raw data of the wild type (WT) and reporter (Cdc22-YFP) strains. (B) Quantification of the red fluorescence by flow cytometry of the strains indicated on the right. (C) Micrographs of the strains indicated on the left. The reporter strain in a wild type background in indicated (-). (D) Quantification of the parameters indicated in the inset in wild type (WT), Δyox1, Δrep2 and Δres1 strains by FACS, quantitative Western Blot (WB) and Q-PCR. Values were normalized to the wild type strain and plotted as mean ±SD of biological triplicates.
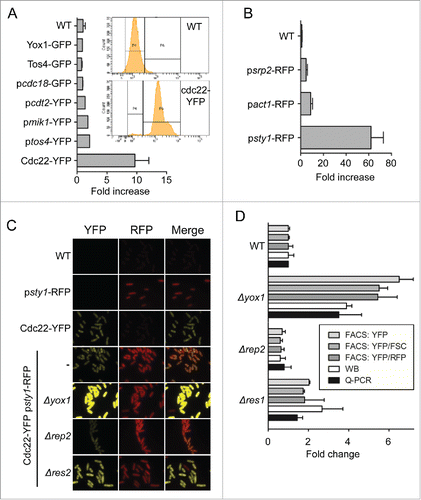
As a proof-of-concept that the Cdc22-YFP reporter could measure changes in MBF activity, we integrated both reporters (Cdc22-YFP and psty1-RFP) in the same strain. Then we deleted known regulators of MBF activity (the repressor Yox1 or the co-activator Rep2) or non-essential elements of the MBF complex (Res1 or Res2). As expected, deletion of the repressor Yox1 increased the yellow fluorescence of the cells without affecting the red fluorescence; on the contrary, deleting rep2 decreased the yellow fluorescence (). Next, we wanted to determine if the reporter strain could measure the changes in a quantitative manner that mirrors the changes in the transcription of MBF-dependent genes. To do so, we measured red and yellow fluorescence of the different strains on the cytometer. As shown in , either the yellow fluorescence values alone (YFP, on the FITC channel) or the ratios to the red fluorescence (YFP/RFP) or to the size (YFP/FSC) showed similar values, with the later one displaying higher reproducibility and consequently lower standard variations. Interestingly, the steady-state levels of Cdc22-YFP [measured on quantitative Western blot (WB)] and the mRNA levels (measured by Q-PCR) showed similar increases or decreases to the fluorescence measured on FACS (). This confirmed that our reporter strain (Cdc22-YFP psty1-RFP) satisfactorily reflects the endogenous MBF activity.
Genome-wide screening to isolate genes that regulate MBF activity
To confirm that the Cdc22-YFP reporter could be used in a genome-wide screen to identify MBF regulators, we crossed the reporter strain with the laboratory knockout collection of chromatin modifiers, including the histone acetylases (HATs) Δhat1, Δgcn5 and Δrtt109, the histone deacetylases (HDACs) Δhos2, Δsir2, Δhst2, Δhst4 and Δclr3, the histone methyltransferases Δset1 and Δset2, and several other general chromatin remodelers (). To test the reproducibility of the process when the strains were grown in 96-well plates, we analyzed the above chromatin-modifier strain mutants and compared the results obtained from cultures grown in individual test tubes to the results obtained from cultures of the same strains grown in 96-well plates. As shown in , there are minor differences in the read-out, independent of how the different strains were processed, either in tubes/flasks or in 96-well plates. Among the 20 strains that we tested, we only detected a clear effect on the YFP/FSC or YFP/RFP in Δnrm1, Δyox1 (both not shown in the plot), Δrep2, Δres1, Δres2 and Δhst4. We were expecting to observe changes in the fluorescence read-out from the MBF mutants (the first 5 strains); we could also detect a significant increase in the strain lacking Hst4, an HDAC from the sirtuin family,Citation15,16 suggesting that this HDAC could be involved in the repression of MBF-dependent transcription. However, it should be noted that the other sirtuins (Sir2 and Hst2) had no effect in the YFP/FSC or YFP/RFP ratios.
Figure 2. Genome-wide screening to identify regulators of MBF activity. (A) Cartoon representing the scheme followed to analyze the mini-library with the chromatin modifiers mutants, either on 96-well plates or grown in individual tubes. (B) Quantification of the YFP/FSC ratio of the strains indicated on the left bearing the Cdc22-YFP reporter. For the 96-well plates, values are plotted as mean ±SD of biological triplicates. (C) Plot of FITC (YFP fluorescence) vs FSC corresponding to the 2792 strains of the knockout collection. Red dots correspond to the top and bottom 1st percentile and green dots to the top and bottom 5th percentile of the FITC/FSC ratio. The position of Δyox1, Δnorm1 and Δrep2 is indicated. (D) GO classification of the 140 strains with lower YFP/FSC ratio, represented as fold enrichment versus the whole knock-out collection. (E) Same as (D) for the 139 strains with higher YFP/FSC ratio.
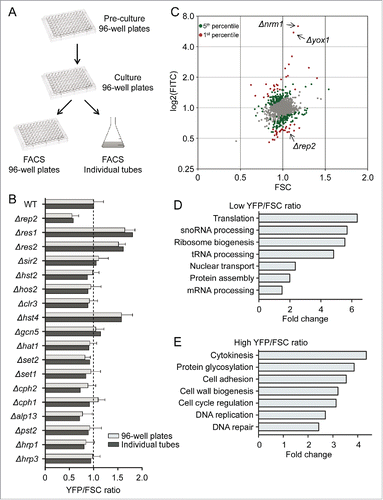
Next, we crossed in the reporter strain with the haploid gene deletion collection from Bioneer, containing 3005 different strains.Citation17 High throughput crossing of the reporter strain with the deletion library in 96-well plates followed by direct plating on selective media allowed the isolation of haploid strains, each one with a single gene deletion and the Cdc22-YFP and the psty1-RFP reporters. Of the total collection, we were able to cross and measure a YFP/FSC ratio in 2792 strains, which represents 92.9% of the original strain collection. At least 2 different biological replicates of each strain were processed and analyzed through the cytometer. Since all the 96-well plates were grown and processed through the cytometer on different days and the laser of the cytometer has a considerable variation in its level of sensitivity from day to day, we decided to include a Δyox1, a Δrep2 and a wild type strain in each plate. These strains were used as quality control after normalization of each plate. Then, the YFP (FITC) value of each of the 2792 strains was plotted against the FSC value (). We selected the top and the bottom 1st percentile of the YFP/FSC ratio and plotted the corresponding strains marked as red dots in . This representation was selected since it helped to discriminate strains with high fluorescence values caused because they were large cells and consequently accumulated more YFP (and not because they had a strong induction of MBF activity); or the opposite: cells with low fluorescence values because they were small (see gray dot with low FITC values in the bottom left quadrant of ). Thus, for each strain we can discriminate the contribution to the YFP/FSC value of both, transcriptional levels (YFP value) and cell size (FSC value). Taking into consideration the values of the 2792 analyzed strains, we obtained a mean YFP/FSC value of 1.00, with a standard deviation of ±0 .21. Thus, we decided to focus on the top and bottom 1st percentile with values that were over 2-fold SD (over 1.55 or below 0.60), reducing the putative hits to 17 different strains with lower MBF activity () and 32 strains with higher MBF activity (). It caught our attention that among the 17 strains with lower YFP/FSC ratios, there was an enrichment of strains in which translation efficiency could be hampered (including mutants of Elongator, ribosomal proteins and tRNA modification pathway). In contrast, among the group of strains with higher YFP/FSC ratios, there was an apparent enrichment of cell cycle mutants. To know exactly if there was such bias to different types of mutants, we extended the list of genes in both categories to the bottom 140 and the top 139 (which represent the 5th percentile; green dots in ) and used Gene Ontology (GO) system to assign genes to a specific biological process. This revealed that among the strains with low YFP/FSC ratio there was a clear enrichment of genes involved in Translation, Ribosome biogenesis, tRNA processing and Protein assembly (), while among the strains with high YFP/FSC ratio there was an enrichment for the terms of Cytokinesis, Cell cycle regulation, DNA replication and DNA repair ().
Table 1. Strains with down-regulated MBF activity.
Table 2. Strains with up-regulated MBF activity.
tRNA methylation and Elongator mutants have impaired MBF-dependent transcription
Given the previous observation indicating that several mutants of the tRNA methylation pathway had low YFP/FSC ratio, we decided to investigate if this pathway was indeed affecting MBF-dependent transcription. As shown in , several mutant strains in the pathway involved in tRNA methylation had low YFP/FSC values (marked as red dots in the plot). To confirm this possibility we prepared RNA from asynchronous cultures of wild type (WT) cells, Δyox1 and Δrep2 and from several mutants of the tRNA methylation pathway. As shown in , there was an overall decrease in the level of MBF-dependent transcription in Δtrm112, Δmtq2 and Δtrm9 cells, measured as the amount of cdc18 mRNA. This effect was not so clear in the other mutants that we tested (Δtrm11, Δlys9 and Δbud23). This fact was not completely surprising, since Trm112 dimerizes with another protein (either Trm9 or Mtq2) to form an active methylase that modifies specific nucleotides in tRNAs.Citation19 However, and despite these strains displaying decreased MBF activity (measured with the reporter strain or as the level of cdc18 transcript), none of these mutants were sensitive to hydroxyurea (HU) (). One possible hypothesis that could explain the impaired MBF-dependent transcription in the tRNA methylation mutants could be that a specific activator of MBF activity (i.e. Rep2) could have impaired translation efficiency. In fact, in a genome-wide analysis it was recently proposed that Rep2 could present low levels of translation efficiency in some mutants of the pathway (27% to 31%, compared to a wild type strain).Citation18 We decided to test whether in a Δtrm112 strain there was a limited amount of Rep2. As shown in , we could not observe any difference in the amount of Rep2 between a wild type and a Δtrm112 strain.
Figure 3. tRNA methyltransferases and Elongator activate MBF. (A) Plot of FITC (YFP fluorescence) vs FSC corresponding to the 2792 strains of the knockout collection with the values of the knock-out strains of genes involved in tRNA methylation highlighted in red. (B) Total RNA from wild type (WT), Δyox1, Δrep2, Δtrm112, Δmtq2, Δtrm9, Δtrm11, Δlys9 and Δbud23 strains was analyzed by Northern blot. Hybridization with an actin (act1) probe and the staining of rRNA is shown as loading control. FI: Signal was quantitated and normalized relative to the signal of the wild type strain. (C) Spot assay of the same strains by serial dilution and growth in rich media or in media with 5 mM HU. Plates were incubated at 30°C for 3–4 d (D) TCA extracts from wild type (WT) and Δtrm112 strains expressing Rep2-HA were immunoblotted with α-HA to detect Rep2. Ponceau staining is shown as loading control. (E) Same as in (A), highlighting knockout strains of Elongator. (F) Total RNA from wild type (WT), Δyox1, Δrep2, Δiki3 and Δctu1 strains was analyzed by Northern blot. act1 hybridization and rRNA are shown as loading controls. FI: Signal was quantitated and normalized relative to the signal of the wild type strain.
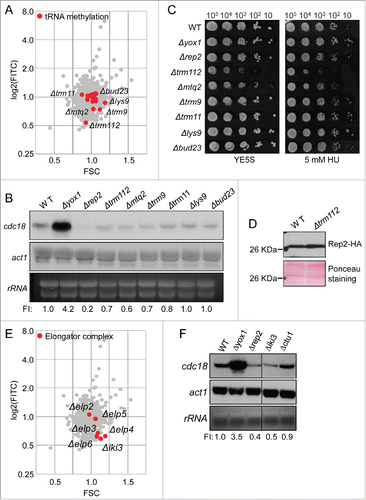
Related to the tRNA modifying enzymes, Elongator complex also promotes efficient translation. We recently reported that the activity of this complex was required for proper tolerance to H2O2 stress and that this effect was mediated by enhanced translation of the transcription factor Atf1.Citation19 From the 6 subunits of the Elongator complex, only Δelp2 and Δelp5 had wild type YFP/FSC ratios, with Δiki3/Δelp1 and Δelp4 having the lowest YFP/FSC ratios (0.52) of all the strains that we tested from the knock-out collection, even below the strain lacking the known MBF co-activator Rep2 (0.55) ( and ). To confirm that the effect measured with the reporter strain was on MBF-dependent transcription, we isolated RNA from wild type, Δyox1 and Δrep2 cells as well as from Δiki3/Δelp1 and Δctu1 cells. We used Δctu1 cells since the Ctu1-Ctu2 complex catalyzes the thiolation at carbon 2 of U34 (s2U34) Citation20 and the modification of specific tRNAs is sequentially made by Trm112 complex and the Ctu1-Ctu2 complex. In fact, although Δctu1 is not included in containing the lowest YFP/FSC strains from our screening, Δctu1 strain had also a low YFP/FSC ratio (0.66). As shown in , we observed a clear reduction of MBF-dependent transcription in Δiki3/Δelp1 cells, similar to the level observed in Δrep2 cells.
COP9/signalosome mutants have induced MBF activity
Among the mutants with higher YFP/FSC ratio we found Δnrm1 and Δyox1 with the highest ratio (5.39 and 4.91, respectively), as expected. We observed that several mutants of COP9/signalosome had a high YFP/FSC ratio, including Δcsn1 and Δcsn2. Interestingly, we also detected increased YFP/FSC ratios in mutants of the COP9-regulated E3 ubiquitin ligase complex Cul4-Ddb1Cdt2, Δcdt2 and Δddb1 ( and ). To confirm that the effect was directly on MBF activity, we prepared RNA from asynchronous cultures of wild type cells, Δyox1 and Δrep2 (as positive and negative regulators of MBF activity) and from several mutants of the COP/signalosome pathway. As shown in , there was an overall increase in the level of MBF-dependent transcription, which was more noticeable in Δddb1 and Δcdt2 cells, measured as the amount of cdc18 mRNA.
Figure 4. COP9/Signalosome down-regulates MBF-dependent transcription. (A) Plot of FITC (YFP fluorescence) vs FSC corresponding to the 2792 strains of the knock-out collection with the knockout strains of COP9/signalosome highlighted in red. (B) Total RNA from cultures of wild type (WT), Δyox1, Δrep2, Δcsn1, Δcsn2, Δddb1, Δcdt2 and Δspd1 strains was analyzed by Northern blot by hybridization with the cdc18 probe. rRNA and act1 are shown as loading controls. FI: Signal was quantitated and normalized relative to the signal of the wild type strain. (C) Yox1 phosphorylation is detected in the strains indicated on top. Wild type (WT) and wild type treated with HU (WT + HU) are shown as phosphorylation control. (D) Spot assay of the same strains (plus Δspd1Δddb1 and Δspd1Δcdt2) analyzed by serial dilution and growth in rich media or in media with 5 mM HU. Plates were incubated at 30°C for 3–4 d (E) Tetrad dissection of Δddb1 crossed with Δrad3 strains. (F) Tetrad dissection of Δddb1 crossed with Δcds1 strains.
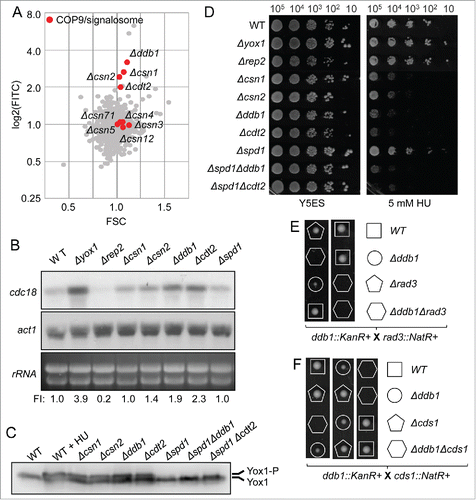
The Cul4-Ddb1Cdt2 complex is implicated in the regulation of at least 2 substrate proteins: Spd1, an inhibitor of ribonucleotide reductase, and Epe1, a heterochromatin regulator.Citation21 Spd1 and Epe1 accumulate in cells lacking Ddb1, Cdt2 or with defective COP9, inducing defects in cell cycle and in heterochromatin silencing. Since the defects in cell cycle are largely rescued by deletion of the ribonucleotide reductase inhibitor, Spd1,Citation22,23 we hypothesized that these strains could have an increased basal activation of the DNA-synthesis checkpoint. One of the consequences of activating Cds1, the effector kinase of this checkpoint, is the phosphorylation of Yox1.Citation11 Yox1 phosphorylation by Cds1 induces its release from MBF and the activation of MBF-dependent transcription, which in turn allows overcoming the arrest imposed by the nucleotide depletion. To test this possibility we determined the status of Yox1 phosphorylation in these mutants. As shown in , Yox1 is phosphorylated in Δcsn1, Δcsn2, Δcdt2 and Δddb1 strains, and to similar level as in a wild type strain treated with hydroxyurea (WT + HU). Furthermore, this phosphorylation is abolished when Spd1 is deleted (in a Δddb1 or in a Δcdt2 background), indicating that in these genetic backgrounds the checkpoint is not activated in basal conditions.
Despite COP9 or E3 ubiquitin ligase complex Cul4-Ddb1Cdt2 mutants have increased MBF activity (measured with the reporter strain or as the level of cdc18 transcript), all these mutants were still highly sensitive to HU (). We also tested the double mutants (Δddb1Δspd1 and Δcdt2Δspd1) in our spot assay, but were unable to increase the resistance to HU treatment (). In spite of this observation, the genetic interaction between E3 ubiquitin ligase complex Cul4-Ddb1Cdt2 mutants and the DNA synthesis checkpoint was clearly confirmed by tetrad dissection. As shown in , double mutants Δddb1Δrad3 and Δddb1Δcds1 are not viable, pointing that the checkpoint needs to be activated in a Δddb1 background or the cells cannot survive to the depletion of nucleotides.
Discussion
The MBF complex is an essential transcription factor that in S. pombe cells controls the expression of the G1-to-S transcription program. Like its metazoan functional analog (pRB/E2F), the regulated activity of this complex is essential for the normal G1/S transition: cells with hypoactive MBF complex are unable to complete S phase while cells with hyperactive MBF show genomic instability.Citation8,24 When DNA replication is challenged (i.e., after treating cells with HU), fission yeast cells activate their effector kinase (Cds1) and, among many other effects, are able to maintain a high level of MBF-dependent transcription.Citation11 To better understand how MBF is activated at the onset of each cell cycle, we have carried out a screening aiming to isolate non-essential genes that regulate MBF activity. The first step was the selection of a reporter system that would mirror MBF activity with high fidelity. Among all the reporter systems that we have assayed, fusing YFP to the carboxy-terminus of Cdc22 was the one that rendered most consistent and reproducible data. Similarly, we also needed a control and we used RFP driven by the promoter of sty1, which is a gene that has high level of transcription that is not cell cycle regulated. In parallel, we also used the size of the cells (FSC in the cytometer), which allows compensating for the accumulation of YFP in large cells (which otherwise would be interpreted as high level of MBF activation).
We managed to cross 2792 different strains in 96-well plates and screen them in an automated cytometer. The screening has rendered 32 mutants with high YFP/FSC ratio and 17 with low ratio. We note that this screening was done measuring steady-state levels of the chimera Cdc22-YFP in asynchronous cultures of fission yeast. This has some limitations, since we are using a protein activity (YFP fluorescence) to measure transcription activity. Also, the fact that both Cdc22 and YFP are long-lived proteins brings an extra layer of difficulty measuring rapid transcriptional changes (especially those that decrease transcription). The use of more dynamic reporters (for example a short-lived Cdc22-YFP) would allow improving the measurement of small or transient changes in MBF activity, especially when MBF is downregulated. Despite this, we have proved that in the reporter strain that we have used there is an excellent correlation between changes in MBF-dependent transcription and the fluorescence of the Cdc22-YFP strain ().
In our screening, we isolated several mutants of the tRNA methylation pathway and from the Elongator complex, which are involved in efficient translation of some mRNAs. Interestingly, we also isolated several ribosomal proteins (Rps and Rpl strains in ), which points in the same direction: efficient translation is required for activation of the MBF complex. In fact, Rep2 is one of the proteins that is supposed to be not well translated in some Elongator mutants.Citation20 However, we do not observe any decrease in the amount of Rep2 that is present in cells of Elongator or tRNA methylation mutants (). Further work will be required to determine how translation efficiency may have an impact on the G1-to-S transcriptional wave.
Finally, we have found that deletions of 2 of the components of the fission yeast COP9/signalosome (Csn1 and Csn2) and also deletions of non-essential components of the COP9-regulated E3 ubiquitin ligase complex Cul4-Ddb1Cdt2, induce MBF-dependent transcription. Although we cannot rule out other mechanisms, it seems that the major impact on MBF regulation is done through the stabilization of the ribonucleotide reductase inhibitor Spd1, which in turn causes, sequentially, the activation of the DNA synthesis checkpoint, phosphorylation of Yox1 and activation of MBF-dependent transcription. Whether COP9 and the E3 ubiquitin ligase complex Cul4-Ddb1Cdt2 impacts on MBF regulation through other mechanisms, still has to be determined.
Material and methods
Strains and media
All S. pombe strains used in this study are listed in . Media were prepared as previously described.Citation25
Table 3. Strains used in this study.
Construction of the reporter strain
Reporter strain JA1845 was generated by crossing JA1818 with P392.Citation26,27 Double-tagged, cycloheximide sensitive and h- meiotic products were selected and crossed to the wild type strain to assess inheritance of rpl42::cyhR. Only parental strains with cycloheximide resistant descendents were selected as reporter strain.
Generation of the reporter library by systematic crossing
PEM2 (Pombe Epistatic Mapping) approach Citation26 was used to systematically cross the Bioneer S. pombe Gene Deletion Library, arrayed in 96-well plates, with the reporter strain JA1845. Plates from the library and a plate containing the JA1845 strain were thawed and spotted into single-well plates containing YE5S + G418 and YE5S + Hygromycin agar, respectively. After growing 2 d at 30°C both plates were crossed in MM-N solid agar using a 96-pin replicator and manual mixing. Mating plates were kept at 25°C for 4 d for sporulation. To eliminate diploid and haploid parental cells, a replica in YE5S + Cycloheximide + G418 was performed and spores were allowed to germinate 2 d at 30°C. Finally, a last round of selection was applied using YE5S + Cycloheximide + G418 + Hygromycin.
Sensitivity analysis
Cells were grown in liquid YE5S media to an OD600 of 0.3. Cultures were 10-fold serially diluted in YE5S and spotted onto drug-free, 5 mM HU or 0,001% MMS containing YE5S plates. Plates were incubated at 30°C for 2-5 d.
RNA analysis
Total RNA from S. pombe rich medium cultures was obtained, processed and transferred to membranes as described previously.Citation11 Membranes were hybridized with the [α-32P]dCTP-labeled cdc18 and act1 probes, containing the complete ORFs.
S. pombe TCA extracts and Western Blot analysis
Modified trichloroacetic acid (TCA) extracts were prepared as previously described.Citation28 Yox1-13Myc was immunodetected with polyclonal anti-Myc antibody (Sigma).
Flow cytometry
Sample preparation
Strains were inoculated in a 96-well plate with 200μl/well of YE5S+G418 using a 96 pin replicator (V&P Scientific). In each plate, rpd3-13Myc, Δyox1 and Δrep2, previously crossed with JA1845, were added with a pipette tip in empty wells H2, H3 and H12, as controls. Cultures were allowed to saturate by growing at 30°C during 24 h with no agitation. An approximate 1/50 dilution was then performed by pinning the saturated cultures into a U-shaped 96-well plate with 150μl/well of liquid YE5S. Plates were sealed with an impermeable membrane (Thermo Scientific) and kept at 30°C O/N with agitation (1000 rpm) to prevent sedimentation. Previous to flow cytometry acquisition, cultures were diluted with a 96 thick pin replicator to obtain a final concentration of approximately OD600 0.025 (0.5 × 106 cells/sec).
Acquisition
BD FACSCanto™ flow cytometer with a BD High Throughput Sampler (HTS) was used for YFP quantification. YFP was excited at 488 nm and detected using a 530/30 band-pass and 502 LP emission filter. Population of interest was obtained by hierarchical gating using i) forward (FSC) and side (SSC) light scattering ii) FSC-A against FSC-H to exclude debris and cell clumps iii) FITC-A and PerCP-Cy5-5-A. For each well, 85 ul of sample were mixed 5 times at 90 ul/s. 60 ul were then analyzed at 2 ul/sec and washed with 800 ul of FACS Flow. Ten.000 events were recorded for each well at ∼1 × 106 events/sec. Data acquisition and processing was performed with BD FACSDiva Software 6.0.
Data processing
YFP/FSC (FITC/FSC) ratios for each well were calculated using raw FSC and FITC median values of the final gated population. To avoid plate-to-plate variation, FITC/FSC ratios of each well were normalized to the mean FITC/FSC ratio of the corresponding plate. 700 events were considered as the minimum threshold of counted events for trustable results. Each plate was analyzed in 2 separate replicas, and the mean of the duplicates was applied for each well to obtain the final FSC, FITC, and FITC/FSC normalized values. 99th percentile was calculated to define a list of “High ratio” and “Low ratio” hits, which included 32 and 17 genes respectively (with values over 1.55 or below 0.60). We also calculated the 99th percentile to produce an extended list of “High ratio” and “Low ratio” hits, which included 139 and 140 genes respectively, which was used for the Gene ontology assignment.
Gene ontology
GO Slimmer was performed with AmiGO 1.8 using the GO Slim Terms list provided by PomBase. Input lists were filtered using PomBase as database filter to reduce possible ambiguity by excluding gene products not found in the database. All Evidence Codes were applied, including IEA (Inferred from Electronic Annotation). For “All genes,” “High ratio,” and “Low ratio,” 592/2792, 14/139 and 12/140 genes were respectively excluded for GO Slimmer calculation. To obtain fold enrichment values of each GO Slim Term for “High ratio” and “Low ratio” lists, background correction was applied by normalization with GO Slimmer results of all genes included in the screening.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are very thankful to Nevan Krogan for providing strain P392, Rafael Carazo-Salas for insightful comments on the screening and Oscar Fornas and staff from the Cytometry Facility at UPF for their help and support. We also thank members of the Oxidative Stress and Cell Cycle Group for help, suggestions and comments. We acknowledge the technical support of Mercè Carmona.
Funding
This work was supported by grants from the Spanish Ministerio de Economia y Competitividad (BFU2012-31939 and BFU2015-66347-P), PLAN E and Feder. E. H. is recipient of an ICREA Academia Award (Generalitat de Catalunya, Spain).
References
- Nurse P, Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature 1981; 292:558-60; PMID:7254352; http://dx.doi.org/10.1038/292558a0
- Caligiuri M, Beach D. Sct1 functions in partnership with Cdc10 in a transcription complex that activates cell cycle START and inhibits differentiation. Cell 1993; 72:607-19; PMID:7916653; http://dx.doi.org/10.1016/0092-8674(93)90079-6
- Lowndes NF, McInerny CJ, Johnson AL, Fantes PA, Johnston LH. Control of DNA synthesis genes in fission yeast by the cell-cycle gene cdc10+. Nature 1992; 355:449-53; PMID:1734281; http://dx.doi.org/10.1038/355449a0
- Zhu Y, Takeda T, Nasmyth K, Jones N. pct1+, which encodes a new DNA-binding partner of p85cdc10, is required for meiosis in the fission yeast Schizosaccharomyces pombe. Genes Dev 1994; 8:885-98; PMID:7926774; http://dx.doi.org/10.1101/gad.8.8.885
- Rustici G, Mata J, Kivinen K, Lio P, Penkett CJ, Burns G, Hayles J, Brazma A, Nurse P, Bahler J. Periodic gene expression program of the fission yeast cell cycle. Nat Genet 2004; 36:809-17; PMID:15195092; http://dx.doi.org/10.1038/ng1377
- Peng X, Karuturi RK, Miller LD, Lin K, Jia Y, Kondu P, Wang L, Wong LS, Liu ET, Balasubramanian MK, et al. Identification of cell cycle-regulated genes in fission yeast. Mol Biol Cell 2005; 16:1026-42; PMID:15616197; http://dx.doi.org/10.1091/mbc.E04-04-0299
- Ayté J, Schweitzer C, Zarzov P, Nurse P, DeCaprio JA. Feedback regulation of the MBF transcription factor by cyclin Cig2. Nat Cell Biol 2001; 3:1043-50; http://dx.doi.org/10.1038/ncb1201-1043
- Caetano C, Limbo O, Farmer S, Klier S, Dovey C, Russell P, de Bruin RA. Tolerance of deregulated G1/S transcription depends on critical G1/S regulon genes to prevent catastrophic genome instability. Cell Rep 2014; 9:2279-89; PMID:25533348; http://dx.doi.org/10.1016/j.celrep.2014.11.039
- Ivanova T, Gomez-Escoda B, Hidalgo E, Ayte J. G1/S transcription and the DNA synthesis checkpoint: common regulatory mechanisms. Cell Cycle 2011; 10:912-5; PMID:21325896; http://dx.doi.org/10.4161/cc.10.6.14963
- Purtill FS, Whitehall SK, Williams ES, McInerny CJ, Sharrocks AD, Morgan BA. A homeodomain transcription factor regulates the DNA replication checkpoint in yeast. Cell Cycle 2011; 10:664-70; PMID:21304269; http://dx.doi.org/10.4161/cc.10.4.14824
- Gómez-Escoda B, Ivanova T, Calvo IA, Alves-Rodrigues I, Hidalgo E, Ayté J. Yox1 links MBF-dependent transcription to completion of DNA synthesis. EMBO Rep 2011; 12:84-9; http://dx.doi.org/10.1038/embor.2010.187
- Ivanova T, Alves-Rodrigues I, Gomez-Escoda B, Dutta C, DeCaprio JA, Rhind N, Hidalgo E, Ayte J. The DNA damage and the DNA replication checkpoints converge at the MBF transcription factor. Mol Biol Cell 2013; 24:3350-7; PMID:24006488; http://dx.doi.org/10.1091/mbc.E13-05-0257
- Kiang L, Heichinger C, Watt S, Bahler J, Nurse P. Cyclin-dependent kinase inhibits reinitiation of a normal S-phase program during G2 in fission yeast. Mol Cell Biol 2009; 29:4025-32; PMID:19487461; http://dx.doi.org/10.1128/MCB.00185-09
- Dutta C, Patel PK, Rosebrock A, Oliva A, Leatherwood J, Rhind N. The DNA replication checkpoint directly regulates MBF-dependent G1/S transcription. Mol Cell Biol 2008; 28:5977-85; PMID:18662996; http://dx.doi.org/10.1128/MCB.00596-08
- Haldar D, Kamakaka RT. Schizosaccharomyces pombe Hst4 functions in DNA damage response by regulating histone H3 K56 acetylation. Eukaryot Cell 2008; 7:800-13; PMID:18344406; http://dx.doi.org/10.1128/EC.00379-07
- Freeman-Cook LL, Sherman JM, Brachmann CB, Allshire RC, Boeke JD, Pillus L. The Schizosaccharomyces pombe hst4(+) gene is a SIR2 homologue with silencing and centromeric functions. Mol Biol Cell 1999; 10:3171-86; PMID:10512858; http://dx.doi.org/10.1091/mbc.10.10.3171
- Kim DU, Hayles J, Kim D, Wood V, Park HO, Won M, Yoo HS, Duhig T, Nam M, Palmer G, et al. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat Biotech 2010; 28:617-23; http://dx.doi.org/10.1038/nbt.1628
- Bauer F, Matsuyama A, Candiracci J, Dieu M, Scheliga J, Wolf DA, Yoshida M, Hermand D. Translational control of cell division by Elongator. Cell Rep 2012; 1:424-33; PMID:22768388; http://dx.doi.org/10.1016/j.celrep.2012.04.001
- Fernandez-Vazquez J, Vargas-Perez I, Sanso M, Buhne K, Carmona M, Paulo E, Hermand D, Rodriguez-Gabriel M, Ayte J, Leidel S, et al. Modification of tRNA(Lys) UUU by elongator is essential for efficient translation of stress mRNAs. PLoS Genet 2013; 9:e1003647; PMID:23874237; http://dx.doi.org/10.1371/journal.pgen.1003647
- Dewez M, Bauer F, Dieu M, Raes M, Vandenhaute J, Hermand D. The conserved Wobble uridine tRNA thiolase Ctu1-Ctu2 is required to maintain genome integrity. Proc Natl Acad Sci U S A 2008; 105:5459-64; PMID:18391219; http://dx.doi.org/10.1073/pnas.0709404105
- Liu C, Powell KA, Mundt K, Wu L, Carr AM, Caspari T. Cop9/signalosome subunits and Pcu4 regulate ribonucleotide reductase by both checkpoint-dependent and -independent mechanisms. Genes Dev 2003; 17:1130-40; PMID:12695334; http://dx.doi.org/10.1101/gad.1090803
- Bayne EH, Bijos DA, White SA, de Lima Alves F, Rappsilber J, Allshire RC. A systematic genetic screen identifies new factors influencing centromeric heterochromatin integrity in fission yeast. Gen Biol 2014; 15:481; http://dx.doi.org/10.1186/s13059-014-0481-4
- Liu C, Poitelea M, Watson A, Yoshida SH, Shimoda C, Holmberg C, Nielsen O, Carr AM. Transactivation of Schizosaccharomyces pombe cdt2+ stimulates a Pcu4-Ddb1-CSN ubiquitin ligase. EMBO J 2005; 24:3940-51; PMID:16252005; http://dx.doi.org/10.1038/sj.emboj.7600854
- Ayte J, Leis JF, Herrera A, Tang E, Yang H, DeCaprio JA. The Schizosaccharomyces pombe MBF complex requires heterodimerization for entry into S phase. Mol Cell Biol 1995; 15:2589-99; PMID:7739540; http://dx.doi.org/10.1128/MCB.15.5.2589
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Meth Enzymol 1991; 194:795-823; PMID:2005825; http://dx.doi.org/10.1016/0076-6879(91)94059-L
- Roguev A, Bandyopadhyay S, Zofall M, Zhang K, Fischer T, Collins SR, Qu H, Shales M, Park HO, Hayles J, et al. Conservation and rewiring of functional modules revealed by an epistasis map in fission yeast. Science 2008; 322:405-10; PMID:18818364; http://dx.doi.org/10.1126/science.1162609
- Roguev A, Wiren M, Weissman JS, Krogan NJ. High-throughput genetic interaction mapping in the fission yeast Schizosaccharomyces pombe. Nature Meth 2007; 4:861-6; http://dx.doi.org/10.1038/nmeth1098
- Vivancos AP, Castillo EA, Biteau B, Nicot C, Ayte J, Toledano MB, Hidalgo E. A cysteine-sulfinic acid in peroxiredoxin regulates H2O2-sensing by the antioxidant Pap1 pathway. Proc Natl Acad Sci U S A 2005; 102:8875-80; PMID:15956211; http://dx.doi.org/10.1073/pnas.0503251102
