ABSTRACT
Plant-derived molecules showing antineoplastic effects have recently gained increased attention as potential adjuvants to traditional therapies for various cancers. Cerrado biome in Brazil contains high floral biodiversity, but knowledge about the potential therapeutic effects of compounds derived from that flora is still limited. The present study investigated the antineoplastic activity of Erythroxylum daphnites Mart., a Brazilian native plant from Cerrado biome, in the SCC-9 oral squamous cell carcinoma cell line. Cells were treated with various concentrations of hexane extract of Erythroxylum daphnites leaves (EDH) and assessed for cytotoxicity, proliferation, and apoptosis. Thin layer chromatography was conducted to characterize the substances present in EDH. Our results showed that EDH exerted anti-proliferative effects in SCC-9 cells by stabilizing the cell cycle at G1 phase in association with reduced intracellular levels of cyclins D and E and increased level of p21. EDH also demonstrated pro-apoptotic properties, as shown by an increased expression of caspase-3. Triterpenes were the major constituents of EDH. Our findings demonstrated a cytotoxic effect of EDH against SCC-9 cells in vitro mediated by the restraint of cellular proliferation and induction of apoptosis. Taken together, these findings support EDH constituents as potential therapeutic adjuvants for oral cancer.
Introduction
Head and neck squamous cell carcinoma (HNSCC) comprises tumors of the oral cavity, oropharynx, larynx, and pharynx.Citation1 Almost half of HNSCCs occur in the oral cavity, and the most common histopathological type is oral squamous cell carcinoma (OSCC).Citation2 Most HNSCCs develop in the upper aero-digestive epithelium after exposure to carcinogens such as tobacco and alcohol.Citation3-6 Moreover, human papillomavirus has also been involved as an etiologic agent in a subset of these cancers.Citation7 In Brazil, there is estimated to be approximately 18,150 new cases of HNSCC in men and 4,780 cases in women every year. These findings suggest a risk of 11.54/100,000 for men and 3.92/100,000 for women of developing cancers of the oral cavity.Citation8
Despite the high incidence of HNSCC, the effectiveness of the available treatments remains limited and patients with recurrent or metastatic HNSCC have a poor prognosis.Citation9 The therapeutic options include surgery for resection of the tumor area and radiotherapy/chemotherapy either alone or in combination.Citation10 For patients who may not undergo any of these treatments, the only alternative is to provide palliative support and care to improve their quality of life.Citation4,9 Moreover, the positive and adverse effects of these treatments should be carefully considered before recommending a specific treatment for HNSCC.Citation11
The use of plants for drug development is not a new concept. Many existing drugs are derived from plants, such as camptothecin, vincristine, vinblastine, taxol, podophyllotoxin, and combretastatin, among others.Citation12 Despite a large number of existing anticancer drugs of natural origin, the pharmacological potential of many plant species has not yet been fully explored. The Erythroxylaceae family comprises 4 genera containing a total of 240 different species that are found endemically in countries such as Venezuela, Brazil, and Madagascar.Citation13 Brazil holds the greatest diversity of Erythroxylum species.Citation14 We previously demonstrated that hexane extracts of Erythroxylum daphnites (EDH) show moderate cytotoxic activity against oral squamous cell carcinoma, which was supra-additive when associated with radiotherapy.Citation15 Therefore, this species can be a strong candidate for the development of new anticancer drugs. The aims of this study were to investigate the molecular mechanisms by which hexane extract of Erythroxylum daphnites displayed antiproliferative and proapoptotic effects in oral cancer cells.
Results
EDH shows selective cytotoxicity against SCC-9 cells
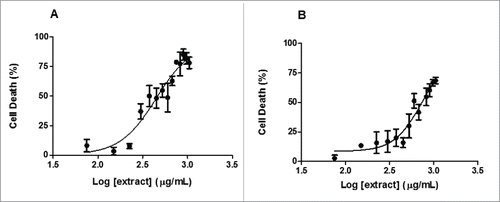
To identify the cytotoxic concentration of EDH, a dose-response curve was performed. The concentration of “0 µg/mL” (addition of solvent only) was considered to have 100% viability, and the viability of other concentrations were expressed as relative values. As we can see, EDH demonstrated moderate cytotoxicity in SCC-9 cells in a dose response manner. The concentration resulting in a 50% reduction in cell viability (IC50) after 24 h of treatment was calculated as 448.9 µg/mL (). For comparison, HaCaT cells, an immortalized keratinocyte cell line, were treated with EDH and presented an IC50 of 737.1 µg/mL (). Based on these values, the tumor selective index (TSI) was calculated as 1.64, showing that EDH was more selective for OSCC cells than for keratinocytes.
Figure 1. Dose-response curves showing IC50 of hexane extract of Erythroxylum daphnites (EDH) on inhibition of both SCC-9 (tongue carcinoma cells) and HaCaT (human Keratinocyte cells) cell viability. Dose-response curves were assessed by MTT assay in SCC-9 (A) and HaCat cells (B) after 24 h of treatment with increased concentration of EDH. The results represent the percentage of dead cells in the presence of different doses of EDH. They are representative of at least 3 independent experiments in triplicate and show the mean ± SEM.
EDH reduces cell proliferation by inducing G1 cell cycle arrest in SCC-9 cells
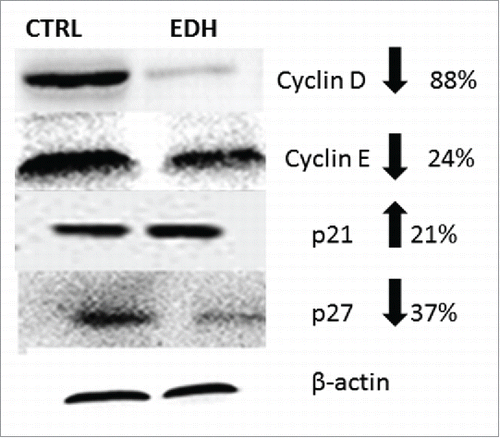
We next evaluated the effects of EDH on cell proliferation by BrdU assay and the cell cycle distribution by flow cytometric analysis. The BrdU cell proliferation assay detects BrdU incorporated into cellular DNA during S phase using an anti-BrdU antibody. As noted, EDH significantly reduced SCC-9 cell proliferation by approximately 31% after 12 h of treatment (P < 0.01 vs control) (). Accordingly, when compared with the corresponding proportions in untreated control cells, EDH resulted in a significant increase in the proportions of cells in the G0 and G1 phases (75%) and a clear reduction in the proportion in S phase (21%) ( and B). To further characterize the effects of EDH on the cell cycle, proteins related to the G1-S cell cycle phase transition were quantified by western blot analysis. As shown in , EDH treatment was associated with increased expression level of the CDK inhibitors p21 by 21% and decreased levels of p27 (37% reduction), cyclin D1 (88% reduction), and cyclin E (24% reduction) compared with their expression levels in untreated control cells. The modification of profile expression of proteins involved in the cell cycle induced by EDH can explain the arrest at G0/G1 phase observed by flow cytometry analysis.
Figure 2. The hexane extract of Erythroxylum daphnites extract (EDH) decreases the proliferation of SCC-9 tongue carcinoma cells. Cell proliferation was measured using BrdU incorporation assay as described in methods. The treatment with EDH significantly decreased the proliferation of SCC-9 cells compared to control. *P < 0.01 vs. control (Student's t test).
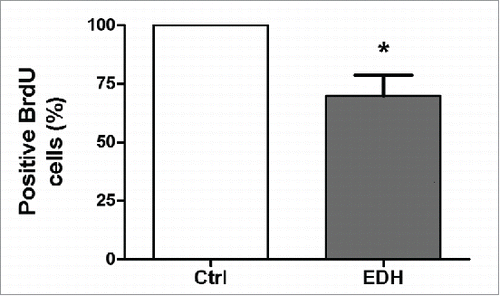
Figure 3. A – Distribution of cells in each stage of the cell cycle after 24 h of treatment with the hexane extract of Erythroxylum daphnites (EDH) (*P < 0.0001 vs. proportion of cells in S-phase). B - Distribution of events labeled with propidium iodide in the histogram, the first gray peak represents events in G0/G1 phase, the second gray peak (right) represents events in G2/M phase, and the hatched region represents the number of events in S phase.
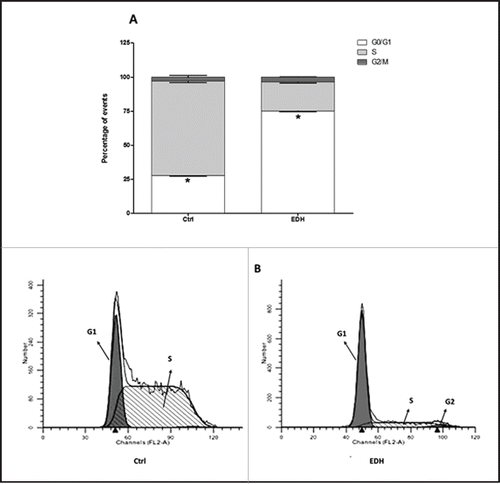
Figure 4. Expression of cell cycle proteins in SCC-9 tongue carcinoma cells assessed by Western blot after 24 hours of treatment with hexane extract of Erythroxylum daphnites (EDH). All these proteins were involved in the transition to G1/S phase of cell cycle. The reduction of expression of cyclin D, cyclin E and p27 and enhanced level of p21 suggest the arrest of cells treated with EDH at G0/G1 phase.
EDH induces apoptosis of SCC-9 cells
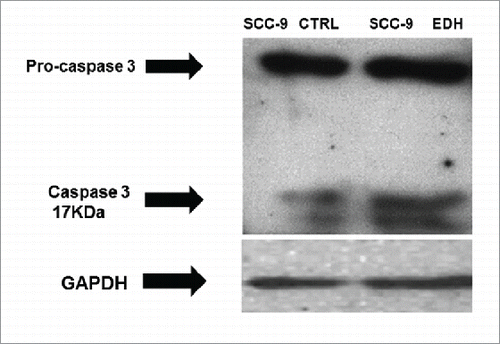
It was previously described that EDH induced 2 different profile of cell death, with treated cells staining positive for both annexin V (indicating apoptosis) and propidium iodide (indicating necrosis).Citation15 To examine the role of apoptosis in EDH-induced SCC-9 cell cycle arrest, we investigated the expression of caspase-3 and pro-caspase by Western blot analysis. Our results demonstrated that the expression of pro-caspase was similar in all analyzed samples, regardless of whether they were treated or not with EDH. In contrast, EDH treatment increased the expression of cleaved caspase (17 kDa) in SCC-9 cells. Collectively, these results suggest the induction of apoptosis in SCC-9 cells treated with EDH ().
Figure 5. Expression of cleavage of Pro-caspase 3 and caspase in SCC-9 tongue carcinoma cells assessed by Western blot after 12 hours of treatment with hexane extract of Erythroxylum daphnites (EDH). The presence of caspase 3 band in SCC-9 cells EDH treated with EDH confirms an apoptotic effect. GAPDH expression was used as housekeeping control (Ctr). The figure represents one of 3 experiments.
Triterpenes are major components of EDH
In order to screen for substances that have cytotoxic activity against SCC-9, the EDH was fractionated into 5 fractions: hexane (EDH-FH); hexane:ethyl acetate (1:1) (EDH-FHE); ethyl acetate:methanol (1:1) (EDH-FEM); methanol (EDH-FM); and ethyl acetate (EDH-FE). Their activities were evaluated by MTT viability assay. As observed in , the EDH fraction of hexane:ethyl acetate (EDH-FHE) and ethyl acetate (EDH-FE) strongly inhibit SCC-9 cell viability. After 24 hours of treatment, only 35.18% and 38.24% respectively remained viable ().
Figure 6. A - Cytotoxicity of hexane extract of Erythroxylum daphnites (EDH) fractions after 24 h of treatment of SCC-9 tongue carcinoma cells. 1 - Fraction hexane:ethyl acetate (1:1) (EDH-FHE); 2 - Fraction ethyl acetate:methanol (EDH-FEM) (1:1); 3 - Fraction ethyl acetate (EDH-FE); 4 - Fraction methanol (EDH-FM); 5 - Fraction hexane (EDH-FH). *P < 0.01 vs. control. B - Chemical structure of compounds found in fraction EDH-FHE. The results show the percentage of cell viability in the presence of different extracts treatment.
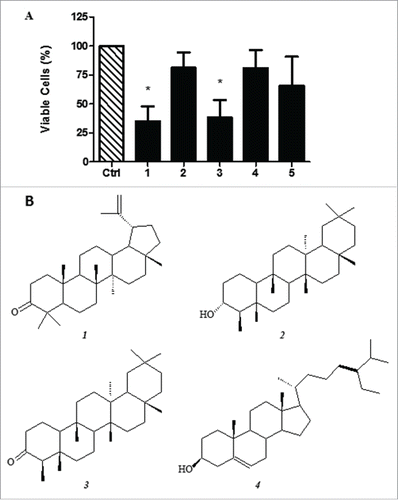
The preliminary analysis of EDH-FHE and EDH-FE by thin layer chromatography showed that both fractions presented a similar chemical profile. In addition, the results suggested the presence of triterpenes as the major class of compounds. The fraction EDH-FHE was subjected to gas chromatography coupled to mass spectrometry (GC-MS) analysis and found to be constituted by the triterpenes lupenone ( − 1), friedelanol ( − 2), and friedelan-3-one ( − 3) and the steroidal compound β-sitosterol ( − 4), as shown in . Furthermore, methyl esters of fatty acids and derivatives were identified, such as methyl palmitate, methyl stearate, methyl linoleate, methyl 18-methyl nonadecanoate, methyl docosanoate, methyl lignocerate, and methyl hexacosanoate ().
Table 1. Components identified in the fraction EDH-FHE by GC-MS analysis.
Discussion
In recent years, plants derivatives have received much attention for their possible utility in the chemoprevention and treatment of tumors.Citation16-22 Among the medicinal plants used for the treatment or prevention of cancer, only 84 species were subjected to rigorous studies and only 35.71% of these species were subjected to pharmacological studies to test their activity.Citation20 The most frequently cited species are Aloe vera (Aloaceae), Euphorbia tirucalli (Euphorbiaceae), and Tabebuia impetiginosa.Citation20 Cerrado biome covers a large area of the Brazilian territory and has a great potential as a natural source of compounds for the pharmaceutical industry.Citation15,23-25 Many species found in this biome are popularly used for the treatment of various diseases, including cancer. Erythroxylum daphnites, from Cerrado biome, presents cytotoxic activity in OSCC cell lines, as previously described by Elias et al. in 2015.Citation15
Furthermore, the selective cytotoxic effect of EDH is promising, with a TSI (tumor selective index) of 1.64 for SCC-9 compared to keratinocytes. This results is very interesting, since many of the adverse effects caused by chemotherapy treatment are consequences of the poor selectivity of the drugs used. Most chemotherapy drugs target rapidly dividing cells, including healthy cells undergoing mitosis as well as cancer cells.Citation26,27 Therefore, the identification of new agents with a higher selectivity for cancer cells is crucial. Similar results were observed in a study by Horri et al,Citation28 who found that Rhinacanthus nasutus extract was strongly selective for an OSCC cell line when compared to gingival fibroblasts, pulp cells, and cells of the periodontal ligament.
EDH also reduced the proliferation rate of SCC-9 cells to nearly 30%, triggering cell cycle arrest at the G1 phase and reducing the proportion of cells in S phase. These effects might be involved in disrupting SCC-9 proliferation. Similar effects of Sinularia extracts were observed by Wang et al.Citation29 in the SCC-25 OSCC cell line and by Chan et al.Citation30 for the flavonoid apigenin.
Genetic alterations are primarily responsible for the development of cancer; known changes include the uncontrolled expression of oncogenes and tumor suppressor genes, and mutations in genes involved in the apoptotic pathway, which lead to genomic instability and consequently progression to a neoplasm.Citation31 Many of these mutations are found in genes that regulate cell cycle G1 phase progression.Citation32,33 Once the cell progresses from G1 to S phase; it is committed to DNA replication and cell division.Citation33 Apoptosis is a fundamental mechanism in various stages of development and actively participates in immune system regulation and homeostasis in multicellular organisms. Failure to control apoptosis might be an underlying cause of several diseases, such as neurodegenerative diseases, autoimmune diseases, and cancer.Citation34 Since cancer development is related to dysfunctions of the apoptotic process, the primary objective of conventional chemotherapy and non-conventional antineoplastic agents, such as natural extracts, and homemade teas, is the induction of cell death in malignant cells by reactivating the apoptotic mechanism.Citation35
Cyclins and cyclin-dependent kinases (CDKs) are primarily responsible for regulating the progression of the cell cycle from G0 to G1 phase and subsequently through the S, G2, and M phases. Changes in the protein levels of cyclins and CDKs mediate transitions between phases of mitosis and ultimately the generation of a daughter cell that is identical to the mother cell.Citation36 Cell cycle transitions are regulated by checkpoint signaling pathways that monitor cellular integrity and ensure the completion of each phase of the cell cycle before initiating the next phase.Citation37 With a focus on tumors that result from cell cycle deregulation, cell cycle specific antineoplastic agents have been widely used in clinical practice, including antimetabolic and hormonal agents and natural products such as vinca alkaloids and taxol.Citation38,39
The expression of caspase-3 in SCC-9 cells that was induced by treatment with EDH confirmed the apoptotic effect of this agent. However, EDH showed a combined profile of cell death induction, with treated cell cultures showing increased proportions of cells staining for annexin V alone, propidium iodide alone, and both markers together when analyzed by flow cytometry. These results suggested that EDH has multiple actions in oral squamous cell carcinoma, involving a rapid induction of apoptosis and progression to necrosis after a longer duration. A similar effect was observed by Chia et al.,Citation16 who found that an extract of Toona sinensis leaves induced death either by apoptosis alone or by a combination of apoptosis and necrosis, depending on the cell type. Other studies have emphasized the importance of apoptosis induction by candidate anticancer agents and the increased use of caspase-3 labeling to establish its occurrence.Citation30,40-42
Once treated with EDH, SCC-9 OSCC cells presented changes in their expression of the proteins involved in cell cycle transitions. Compared with the corresponding measurements in control cells, the expression levels of cyclins D and E and p27 decreased in EDH-treated cells, while that of p21 was increased. The activity of paclitaxel, a drug widely used to treat cancer, was positively correlated with cyclin B1/CDC2 activity, prolonged mitotic arrest, and Bcl-2 phosphorylation. In contrast, carboplatin caused cell cycle arrest before mitosis in HNSCC cells.Citation37 Treatment of HNSCC cells with metformin also reduced the expression of Cdk2, Cdk4, Cdk6, cyclins D and E, and CDK inhibitors such as p15, p16, p18, and p27.Citation43 At the G1/S phase transition, the proteins p53, pRb, and a host of CDK inhibitors (p21Waf1/Cip1, p27Kip1, p57Kip2, and p16INK4A) are necessary for checkpoint function.Citation44 Cyclin D, encoded by the CCND1 gene, essentially functions as a regulatory subunit of Cdk4 and Cdk6. Cyclin D activity is of fundamental importance for the transition from G1 to S phase.Citation36 Cyclin E targets Cdk2 and the interaction between these proteins is also critical for progression from G1 to S phase. The cyclin E/Cdk2 complex phosphorylates p27 (a cyclin D inhibitor) and induces the expression of cyclin A, which is required for entry into S phase.Citation36,45
Multiple compounds in EDH have shown cytotoxic activities against several cancer cell lines.Citation46-50 Therefore, we hypothesize that these compounds might be responsible, at least in part, for the cytotoxic effect observed in OSCC cells. Lupenone was able to increase tyrosinase mRNA expression via inhibiting the phosphorylation of extracellular signal-regulated kinases 1 and 2 by mitogen-activated protein kinase in B16 murine melanoma cells.Citation49,50 Additionally, lupenone exhibited weak cytotoxicity against human colorectal cancer (HT-29) and mammary breast cancer (MDA-MB) cell lines.Citation46,47 β-sitosterol is one of the most common phytosterols in higher plants and presents several pharmacological activities, including anti-tumor activities.Citation51-54 This compound showed antiproliferative effects in MCF-7 cells, mediated by caspase-induced apoptosis.Citation48 In addition, β-sitosterol inhibited non-metastatic 22Rv1 and metastatic DU145 human prostate cell lines but had no such effect on normal cells.Citation55 Fatty acids have been recognized as cytotoxic agents. The presence of fatty acids and derivatives in Erythroxylum daphnites extract and its fractions also explains the cytotoxicity of these agents against the tested OSCC cell line. Saturated fatty acids (oleic, palmitoleic, palmitic, and stearic acids) can induce hepatocyte lipo-apoptosis by activating the proapoptotic Bcl-2 proteins Bim and Bax, which trigger the mitochondrial apoptotic pathway.Citation56 Stearic, sterculic, and oleic acids were found to inhibit colony formation by 5 human cancer cell lines and 2 non-neoplastic cell lines in a dose-dependent manner. In that study, stearic acid was more active than oleic acid for all cell lines except HT29.Citation57
Our results showed that EDH induced apoptosis in the SCC-9 OSCC cell line. EDH-treated SCC-9 cells were arrested in G1 phase and showed an altered expression of proteins involved in cell cycle progression. These effects were selective to SCC-9 cells. Taken together, EDH appears to be a promising candidate agent for the treatment of OSCC. Triterpenes and fatty acids may contribute to its cytotoxic effect in OSCC cells.
Materials and methods
Plant material
Leaves of Erythroxylum daphnites Mart. were collected from Cerrado biome in the city of Brasília (Brazil) and its surroundings, and deposited in the herbarium of the University of Brasilia [voucher number (UB) 2193]. The leaves were dried at room temperature and powdered in a knife mill. The plant material (40 g) was macerated with hexane (2 L) at room temperature for 7 days (repeated 3 times). After filtration, the solvents were removed under reduced pressure at temperatures below 40°C, furnishing hexane crude extract (EDH) (5.8% yield).
The crude extract (EDH) was subjected to filtration over silica gel 60 (Fluka) and gave 5 fractions, according to the eluent used: hexane (FH, 11.8% yield); hexane:ethyl acetate 1:1 (FHE, 51.5% yield); ethyl acetate (FE, 26.1% yield); ethyl acetate:methanol 1:1 (FEM, 9.2% yield); and methanol (FM, 1.1% yield). The crude extract and fractions were analyzed by thin layer chromatography (Alugram® Sil G, Macherey-Nagel), using a mixture of hexane and ethyl acetate (7:3) as eluent. The chromatogram was revealed by using anisaldehyde reagent.Citation58
The most active fraction (FHE) was subjected to saponification and transesterification reactions, according to Hartman and Lago (1973), with modifications,Citation59,60 resulting in saponified and unsaponified fractions. GC-MS analysis was performed on a GCMS-QP2010 Plus system (IE 70 eV) (Shimadzu) with a Rtx®-5MS column measuring 30 m × 0.32 mm × 0.25 µm, packed with 5% diphenyl and 95% dimethylpolysiloxane. The injection port was maintained at 250°C (splitless mode), and analysis was performed at a temperature gradient increasing from 100°C to 300°C at a rate of 5°C/min. The carrier gas was helium at a flow-rate of 6 mL/min.
Cell culture
SCC-9 cells, a tongue squamous carcinoma cell line, and HaCaT cells, an immortalized keratinocyte cell line, were cultured according to the recommendations of the American Type Culture Collection. SCC-9 cells were grown as monolayers in a mixture of DMEM/Ham's F12 (D2906, Sigma-Aldrich) medium and HaCaT cells were cultured in DMEM. They were supplemented with 10% (v/v) fetal bovine serum (FBS) (12657-029, Gibco, Life Technologies), 1% (v/v) antibiotics solution (P0781, Sigma-Aldrich), and 400 ng/mL hydrocortisone (H0888, Sigma-Aldrich) and maintained at 37°C in a humidified atmosphere 5% of CO2.
Dose response curve for cell viability
Cells (5 × 103) were seeded in 96 well plates and allowed to adhere overnight at 37°C, followed by treatments of cells with different concentrations (1050, 975, 900, 825, 750, 675, 600, 525, 450, 375, 300, 225, 150, 75, and 0 µg/mL) of EDH for 24 h. As a negative control, the solvent used for dilution of the extract was added to the culture medium at the maximum volume used for treatment. MTT reagent (M2128, Sigma-Aldrich) was added to each well and incubated for 4 h at 37°C in the dark. The media was removed and 100 µL of NHCl (1007, Vetec Quimica)/isopropanol (9084-03, J.T. Baker, Avantor Performance Materials) solution was added to each well and incubated for 15 min at room temperature under gentle agitation. Finally, absorbance was measured at 570 nm in a microplate reader. Three independent experiments were performed, each with triplicate measurements. The IC50 value was calculated, and the TSI was established as the ratio of IC50 in the control cells (HaCaT) to that in the neoplastic cells (SCC-9), as previously published by Horri et al.Citation28
Cell proliferation assay (BrdU)
SCC-9 cells were plated at a density of 1 × 104 cells per well in 96 well plates and maintained under optimum conditions. After adherence, cells were washed with phosphate-buffered saline (PBS), and fresh serum-free medium was added to the plated cells for 24 h to induce cell synchronism. Cells were then treated with the IC50 of the extract for 12 h. Finally, BrdU (11647229001, Roche) was added at a final concentration of 10 µM per well, and the cells were incubated for 2 h at 37°C. After the incubation period, medium containing BrdU was removed, and the cells were fixed for 30 min at room temperature with cold 70% (v/v) ethanol. The incorporation of BrdU into proliferating cells was quantified by ELISA as described by the manufacturer. To stop the reaction, 25 µL of a 1 M solution of H2SO4 (320501, Sigma-Aldrich) was added per well, and absorbance was read at a wavelength of 450 nm (reference wavelength 690 nm) using an ELISA reader (Ultramark™ Microplate Imaging System, Bio-Rad).
Cell cycle analysis
Cells were synchronized for 24 h by serum starvation and released by replacement with media containing 10% (v/v) FBS and the tested concentrations of EDH as noted above. After 24 h, cells were collected, fixed in 70% (v/v) ethanol for 30 min, treated with 10 μg/mL of RNAse (Sigma-Aldrich) and stained with 50 μg/mL of propidium iodide (Sigma-Aldrich). The distribution of cells in each cell cycle phase was analyzed with the aid of a FACSCalibur™ flow cytometer (BD Biosciences) equipped with an argon laser and ModFit LT™ software (Verity Software House).
Apoptosis analysis
The apoptosis index was determined by annexin V labeling. Briefly, cells treated for 2 and 6 h with the IC50 of EDH were harvested, washed, and resuspended in binding buffer (10 mM HEPES pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1.8 mM CaCl2) containing annexin V-PE and 7-AAD dyes (BD Biosciences). Apoptosis was analyzed on a FACSCalibur™ flow cytometer equipped with an argon laser (BD Biosciences) and quantified as the number of annexin V-PE positive and 7-AAD negative cells divided by the total number of cells. A minimum of 10,000 events was analyzed for each sample.
Western blot
Cells were washed with cold PBS and lysed in a buffer containing 20 mM Tris-HCl pH 8.0, 137 mM NaCl, 10% (v/v) glycerol, 2 mM ethylenediaminetetraacetic acid, and protease inhibitors. After centrifugation, protein concentrations were measured using a protein assay according to the manufacturer's instructions (Bio-Rad Protein Assay, Bio-Rad). Four micrograms of total proteins per sample were resolved by SDS-PAGE on a 10% (v/v) polyacrylamide gel under reducing conditions and transferred to nitrocellulose membranes. The membranes were blocked with 10% (w/v) non-fat dried milk in PBS containing 0.1% (v/v) Tween® 20, rinsed in the same buffer, and incubated for 2 h with the primary antibodies (). After washing, the protein bands were detected using enhanced chemiluminescence reagent (GE Healthcare).
Table 2. List of primary antibodies.
Statistical analysis
Cytotoxicity results were tested by one-way ANOVA with Tukey's post-hoc multiple comparison test. For the analysis of cell proliferation (BrdU assay) and apoptosis, Student's t-test was applied. Dose-response curves were assessed by non-linear regression of inhibition vs. dose, and the IC50 value was calculated by interpolation. For cell cycle analysis, 2-way ANOVA with the Bonferroni post-hoc test was performed. For all experiments, the control represents cells treated only with the solvent used to dilute EDH. All data were analyzed with Prism® software, version 5.0 (GraphPad Software). Values of p < 0.05 were considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (MCT/CNPq/FNDCT/FAPs/MEC/CAPES/PRO-CENTRO-OESTE N. 031/2010 project 564658/2010–3 and 564208/2010-8), and by Fundação de Amparo à Pesquisa-Distrito Federal (FAP-DF) project 193.000.484/2011.
References
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 2009; 45:309-16; PMID:18804401; http://dx.doi.org/10.1016/j.oraloncology.2008.06.002
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225-49; PMID:19474385; http://dx.doi.org/10.3322/caac.20006
- Amarasinghe HK, Johnson NW, Lalloo R, Kumaraarachchi M, Warnakulasuriya S. Derivation and validation of a risk-factor model for detection of oral potentially malignant disorders in populations with high prevalence. Br J Cancer 2010; 103:303-9; PMID:20628386; http://dx.doi.org/10.1038/sj.bjc.6605778
- Belcher R, Hayes K, Fedewa S, Chen AY. Current treatment of head and neck squamous cell cancer. J Surg Oncol 2014; 110:551-74; PMID:25053506; http://dx.doi.org/10.1002/jso.23724
- Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer 2003; 3:733-44; PMID:14570033; http://dx.doi.org/10.1038/nrc1190
- Scully C, Bagan JV. Recent advances in Oral Oncology. Oral Oncol 2007; 43:107-15; PMID:17275742; http://dx.doi.org/10.1016/j.oraloncology.2006.12.007
- Thavaraj S, Stokes A, Guerra E, Bible J, Halligan E, Long A, Okpokam A, Sloan P, Odell E, Robinson M. Evaluation of human papillomavirus testing for squamous cell carcinoma of the tonsil in clinical practice. J Clin Pathol 2011; 64:308-12; PMID:21345874; http://dx.doi.org/10.1136/jcp.2010.088450
- Brazil. Estimativa 2014 - Incidência do Câncer no Brasil. Rio de Janeiro Ministério da Saúde. Instituto Nacional de Câncer José de Alencar Gomes da Silva (INCA), 2014
- Massa E, Dessi M, Gaspardini G, Saba F, Cherchi V, Mantovani G. Phase II study of vinorelbine/cetuximab in patients with recurrent/metastatic squamous cell carcinoma of the head and neck progressing after at least two chemotherapy regimens. Oral Oncol 2010; 46:818-21; PMID:20920877; http://dx.doi.org/10.1016/j.oraloncology.2010.08.013
- Klug C, Berzaczy D, Voracek M, Millesi W. Preoperative chemoradiotherapy in the management of oral cancer: a review. J Craniomaxillofac Surg 2008; 36:75-88; PMID:18222699; http://dx.doi.org/10.1016/j.jcms.2007.06.007
- Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncol 2009; 45:301-8; PMID:19249237; http://dx.doi.org/10.1016/j.oraloncology.2009.01.004
- Srivastava V, Negi AS, Kumar JK, Gupta MM, Khanuja SP. Plant-based anticancer molecules: a chemical and biological profile of some important leads. Bioorg Med Chem 2005; 13:5892-908; PMID:16129603; http://dx.doi.org/10.1016/j.bmc.2005.05.066
- Daly DC. Erythroxylaceae. In: Smith NP, Mori SA, Henderson A, Stevenson DW, Heald SV, eds. Flowering Plant Families of the American Tropics Princeton University Press/New York Botanical Garden, 2003:143-5
- Oliveira SL, da Silva MS, Tavares JF, Sena‐Filho JG, Lucena HF, Romero MA, Barbosa‐Filho JM. Tropane Alkaloids from Erythroxylum Genus: Distribution and Compilation of 13C‐NMR Spectral Data. Chem Biodiversity 2010; 7:302-26; http://dx.doi.org/10.1002/cbdv.200800290
- Elias ST, Borges GA, Amorim DA, Rego DF, Simeoni LA, Silveira D, Fonseca-Bazzo YM, Paula JE, Fagg CW, Barros IM, et al. Radiation induced a supra-additive cytotoxic effect in head and neck carcinoma cell lines when combined with plant extracts from Brazilian Cerrado biome. Clin Oral Invest 2015; 19:637-46; http://dx.doi.org/10.1007/s00784-014-1289-z
- Chia YC, Rajbanshi R, Calhoun C, Chiu RH. Anti-neoplastic effects of gallic acid, a major component of Toona sinensis leaf extract, on oral squamous carcinoma cells. Molecules 2010; 15:8377-89; PMID:21081858; http://dx.doi.org/10.3390/molecules15118377
- Endringer DC, Valadares YM, Campana PR, Campos JJ, Guimaraes KG, Pezzuto JM, Braga FC. Evaluation of Brazilian plants on cancer chemoprevention targets in vitro. Phytother Res 2010; 24:928-33; PMID:19957245
- Henning SM, Wang P, Heber D. Chemopreventive effects of tea in prostate cancer: green tea versus black tea. Mol Nutrition Food Res 2011; 55:905-20; http://dx.doi.org/10.1002/mnfr.201000648
- Kviecinski MR, Felipe KB, Schoenfelder T, de Lemos Wiese LP, Rossi MH, Goncalez E, Felicio JD, Filho DW, Pedrosa RC. Study of the antitumor potential of Bidens pilosa (Asteraceae) used in Brazilian folk medicine. J Ethnopharmacol 2008; 117:69-75; PMID:18342465; http://dx.doi.org/10.1016/j.jep.2008.01.017
- Melo JG, Santos AG, Amorim ELC, Nascimento SC, Albuquerque UP. Medicinal plants used as antitumor agents in Brazil: an ethnobotanical approach. Evidence-Based Complementary Alternative Med 2011; 2011:365359
- Santos Junior HM, Oliveira DF, Carvalho DA, Pinto JM, Campos VA, Mourao AR, Pessoa C, Moraes MO, Costa-Lotufo LV. Evaluation of native and exotic Brazilian plants for anticancer activity. J Nat Med 2010; 64:231-8; PMID:20127421; http://dx.doi.org/10.1007/s11418-010-0390-0
- Suffredini IB, Paciencia ML, Varella AD, Younes RN. In vitro cytotoxic activity of Brazilian plant extracts against human lung, colon and CNS solid cancers and leukemia. Fitoterapia 2007; 78:223-6; PMID:17346903; http://dx.doi.org/10.1016/j.fitote.2006.11.011
- Mesquita ML, Paula JE, Pessoa C, Moraes MO, Costa-Lotufo LV, Grougnet R, Michel S, Tillequin F, Espindola LS. Cytotoxic activity of Brazilian Cerrado plants used in traditional medicine against cancer cell lines. J Ethnopharmacol 2009; 123:439-45; PMID:19501276; http://dx.doi.org/10.1016/j.jep.2009.03.018
- Elias ST, Salles PM, de Paula JE, Simeoni LA, Silveira D, Guerra EN, Motoyama AB. Cytotoxic effect of Pouteria torta leaf extracts on human oral and breast carcinomas cell lines. J Cancer Res Ther 2013; 9:601-6; PMID:24518703; http://dx.doi.org/10.4103/0973-1482.126454
- Toledo CE, Britta EA, Ceole LF, Silva ER, Mello JC, Dias Filho BP, Nakamura CV, Ueda-Nakamura T. Antimicrobial and cytotoxic activities of medicinal plants of the Brazilian cerrado, using Brazilian cachaca as extractor liquid. J Ethnopharmacol 2011; 133:420-5; PMID:20951786; http://dx.doi.org/10.1016/j.jep.2010.10.021
- Florea AM, Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011; 3:1351-71; PMID:24212665; http://dx.doi.org/10.3390/cancers3011351
- Peres LA, Cunha AD, Jr. Acute nephrotoxicity of cisplatin: molecular mechanisms. J Bras Nefrol 2013; 35:332-40; PMID:24402113; http://dx.doi.org/10.5935/0101-2800.20130052
- Horii H, Suzuki R, Sakagami H, Umemura N, Ueda JY, Shirataki Y. Induction of non-apoptotic cell death in human oral squamous cell carcinoma cell lines by Rhinacanthus nasutus extract. In Vivo 2012; 26:305-9; PMID:22351674
- Wang GH, Chou TH, Lin RJ, Sheu JH, Wang SH, Liang CH. Cytotoxic Effect of the Genus Sinularia Extracts on Human SCC25 and HaCaT Cells. J Toxicol 2009; 2009:634868; PMID:20130779
- Chan LP, Chou TH, Ding HY, Chen PR, Chiang FY, Kuo PL, Liang CH. Apigenin induces apoptosis via tumor necrosis factor receptor- and Bcl-2-mediated pathway and enhances susceptibility of head and neck squamous cell carcinoma to 5-fluorouracil and cisplatin. Biochim Biophys Acta 2012; 1820:1081-91; PMID:22554915; http://dx.doi.org/10.1016/j.bbagen.2012.04.013
- Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer 2001; 1:222-31; PMID:11902577; http://dx.doi.org/10.1038/35106065
- Foster DA, Yellen P, Xu L, Saqcena M. Regulation of G1 Cell Cycle Progression: Distinguishing the Restriction Point from a Nutrient-Sensing Cell Growth Checkpoint(s). Genes Cancer 2010; 1:1124-31; PMID:21779436; http://dx.doi.org/10.1177/1947601910392989
- Masamha CP, Benbrook DM. Cyclin D1 degradation is sufficient to induce G1 cell cycle arrest despite constitutive expression of cyclin E2 in ovarian cancer cells. Cancer Res 2009; 69:6565-72; PMID:19638577; http://dx.doi.org/10.1158/0008-5472.CAN-09-0913
- Park HH. Structural features of caspase-activating complexes. Int J Mol Sci 2012; 13:4807-18; PMID:22606010; http://dx.doi.org/10.3390/ijms13044807
- Sakagami H, Kobayashi M, Chien CH, Kanegae H, Kawase M. Selective toxicity and type of cell death induced by various natural and synthetic compounds in oral squamous cell carcinoma. In Vivo 2007; 21:311-20; PMID:17436582
- Carnero A. Targeting the cell cycle for cancer therapy. Br J Cancer 2002; 87:129-33; PMID:12107831; http://dx.doi.org/10.1038/sj.bjc.6600458
- Coleman SC, Stewart ZA, Day TA, Netterville JL, Burkey BB, Pietenpol JA. Analysis of cell-cycle checkpoint pathways in head and neck cancer cell lines: implications for therapeutic strategies. Arch Otolaryngol Head Neck Surg 2002; 128:167-76; PMID:11843726; http://dx.doi.org/10.1001/archotol.128.2.167
- Johnson IS, Wright HF, Svoboda GH, Vlantis J. Antitumor principles derived from Vinca rosea Linn. I. Vincaleukoblastine and leurosine. Cancer Res 1960; 20:1016-22; PMID:14407465
- Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 1971; 93:2325-7; PMID:5553076; http://dx.doi.org/10.1021/ja00738a045
- Boubaker J, Bhouri W, Sghaier MB, Bouhlel I, Skandrani I, Ghedira K, Chekir-Ghedira L. Leaf extracts from Nitraria retusa promote cell population growth of human cancer cells by inducing apoptosis. Cancer Cell Int 2011; 11:37; PMID:22040460; http://dx.doi.org/10.1186/1475-2867-11-37
- Colquhoun AJ, Venier NA, Vandersluis AD, Besla R, Sugar LM, Kiss A, Fleshner NE, Pollak M, Klotz LH, Venkateswaran V. Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer Prostatic Dis 2012; 15:346-52; PMID:22614062; http://dx.doi.org/10.1038/pcan.2012.16
- Elias ST, Diniz J, Almeida RS, Alvarenga N, Simeoni LA, Silveira D, Ferro E, Guerra EN, Motoyama AB. Cytotoxic effect of tobacco extracts on human oral squamous cell carcinoma cell-line. Oral Oncol 2010; 46:869-73; PMID:20971678; http://dx.doi.org/10.1016/j.oraloncology.2010.09.008
- Sikka A, Kaur M, Agarwal C, Deep G, Agarwal R. Metformin suppresses growth of human head and neck squamous cell carcinoma via global inhibition of protein translation. Cell Cycle 2012; 11:1374-82; PMID:22421144; http://dx.doi.org/10.4161/cc.19798
- Kaufmann WK, Paules RS. DNA damage and cell cycle checkpoints. FASEB J 1996; 10:238-47; PMID:8641557
- Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol 2013; 14:518-28; PMID:23877564; http://dx.doi.org/10.1038/nrm3629
- Ahmad S, Sukari MA, Ismail N, Ismail IS, Abdul AB, Bakar MFA, Kifli N, Ee GC. Phytochemicals from Mangifera pajang Kosterm and their biological activities. BMC Complementary Alternative Med 2015; 15:83; http://dx.doi.org/10.1186/s12906-015-0594-7
- Al Muqarrabun LR, Ahmat N, Aris SRS, Shamsulrijal N, Baharum SN, Ahmad R, Rosandy AR, Suratman MN, Takayama H. A new sesquiterpenoid from scaphium macropodum (Miq.) beumee. Natural Product Res 2014; 28:597-605; http://dx.doi.org/10.1080/14786419.2014.886211
- Chai J, Kuppusamy U, Kanthimathi M. Beta-sitosterol induces apoptosis in MCF-7 cells. Malaysian J Biochem Mol Biol 2008; 16:28-30
- Hata K, Hori K, Takahashi S. Differentiation- and apoptosis-inducing activities by pentacyclic triterpenes on a mouse melanoma cell line. J Nat Prod 2002; 65:645-8; PMID:12027734; http://dx.doi.org/10.1021/np0104673
- Villareal MO, Han J, Matsuyama K, Sekii Y, Smaoui A, Shigemori H, Isoda H. Lupenone from Erica multiflora leaf extract stimulates melanogenesis in B16 murine melanoma cells through the inhibition of ERK1/2 activation. Planta Med 2013; 79:236-43; PMID:23408272; http://dx.doi.org/10.1055/s-0032-1328189
- Yasukawa K, Takido M, Matsumoto T, Takeuchi M, Nakagawa S. Sterol and triterpene derivatives from plants inhibit the effects of a tumor promoter, and sitosterol and betulinic acid inhibit tumor formation in mouse skin two-stage carcinogenesis. Oncol 1991; 48:72-6; http://dx.doi.org/10.1159/000226898
- Zhao Y, Chang SK, Qu G, Li T, Cui H. β-Sitosterol inhibits cell growth and induces apoptosis in SGC-7901 human stomach cancer cells. J Agricultural Food Chem 2009; 57:5211-8; http://dx.doi.org/10.1021/jf803878n
- Sook SH, Lee HJ, Kim JH, Sohn EJ, Jung JH, Kim B, Kim JH, Jeong SJ, Kim SH. Reactive oxygen species‐mediated activation of AMP‐activated protein kinase and c‐Jun N‐terminal kinase plays a critical role in beta‐sitosterol‐induced apoptosis in multiple myeloma U266 cells. Phytotherapy Res 2014; 28:387-94; http://dx.doi.org/10.1002/ptr.4999
- Saeidnia S, Manayi A, Gohari AR, Abdollahi M. The story of β-sitosterol-a review. Eur J Med Plants 2014; 4:590-609; http://dx.doi.org/10.9734/EJMP/2014/7764
- Jourdain C, Tenca G, Deguercy A, Troplin P, Poelman D. In-vitro effects of polyphenols from cocoa and β-sitosterol on the growth of human prostate cancer and normal cells. Eur J Cancer Prevention 2006; 15:353-61; http://dx.doi.org/10.1097/00008469-200608000-00009
- Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem 2006; 281:12093-101; PMID:16505490; http://dx.doi.org/10.1074/jbc.M510660200
- Fermor BF, Masters JR, Wood CB, Miller J, Apostolov K, Habib NA. Fatty acid composition of normal and malignant cells and cytotoxicity of stearic, oleic and sterculic acids in vitro. Eur J Cancer 1992; 28:1143-7; http://dx.doi.org/10.1016/0959-8049(92)90475-H
- Wagner H, Bladt S. Plant drug analysis: a thin layer chromatography atlas. Springer, 1996
- Hartman L, Lago R. Rapid preparation of fatty acid methyl esters from lipids. Lab Practice 1973; 22:475
- Milinsk MC, Matsushita M, Visentainer JV, Oliveira CCd, Souza NED. Comparative analysis of eight esterification methods in the quantitative determination of vegetable oil fatty acid methyl esters (FAME). J Brazilian Chem Society 2008; 19:1475-83; http://dx.doi.org/10.1590/S0103-50532008000800006
