It has been largely anticipated that the incidence of hematological malignancies in the elderly can be traced back to age-associated alterations in both the haematopoietic system and the haematopoietic stem cell (HSC) compartment.Citation1 Thus, although molecular and cellular damage accumulation drives the functional decline of organisms in aging, several conserved signaling pathways influence how rapidly damage accumulates and how organisms respond to it.Citation2 Among them, the insulin/IGF-1 signaling pathway (IIS) is one of the most important determinants of organism lifespan. Prolonged suppression of the IIS pathway promotes longevity, whereas persistent activation shortens lifespan and increases cancer incidence.
We have recently provided experimental support for an unanticipated tumor suppressor activity of AIRAPL in myeloid transformation, connecting altered proteostasis with IIS pathway deregulation and development of myeloproliferative neoplasms (MPN).Citation3,4 AIRAPL is an endoplasmic reticulum (ER) protein that contains a CAAX motif, similar to those present in Ras proteins or nuclear lamins. Currently available functional data on AIRAPL had been obtained from its Caenorhabditis elegans ortologue aip-1. These previous studies had demonstrated the involvement of this protein in proteostasis control during aging.Citation5 However, we have recently found that the inactivation of the mouse Zfand2b gene – encoding AIRAPL − is sufficient to cause cell-autonomous defects in the HSC compartment, giving rise to a myeloid neoplasia that faithfully resembles most of the alterations shown by MPN patients as well as by animal models of this disease. Furthermore, we have demonstrated that subtle changes in AIRAPL levels remarkably affect myeloid hematopoiesis, suggesting a prominent role of this protein in the control of white blood cell formation.
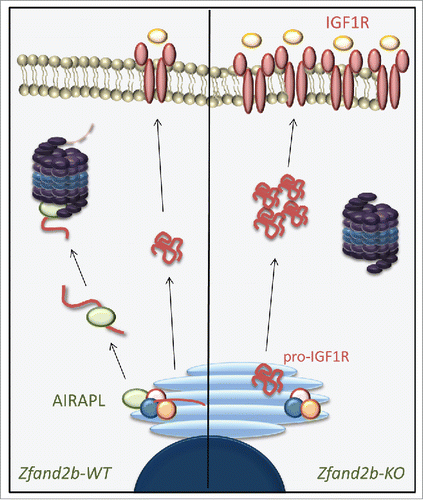
Proteomic analysis performed on cells from Zfand2b-deficient mice revealed that AIRAPL is a central regulator of IGF1R steady-state levels at the ER through proteostasis modulation. The proposed mechanism agrees with recent evidences that have demonstrated the implication of AIRAPL in pre-emptive quality control (PQC) pathway.Citation6 Accordingly, we propose that AIRAPL-dependent PQC activity promotes translocation of IGF1R nascent polypeptides out of the ER and their proteasome-mediated degradation. Although AIRAPL is unlikely to display E3-ligase activity, we have found that its overexpression is sufficient to promote IGF1R ubiquitination. The degradation of IGF1R polypeptides prior to their arrival to the cell surface suppresses excessive IGF-1 signaling ().
Figure 1. Proposed AIRAPL biological function. AIRAPL, encoded by the Zfand2b gene, interacts with newly synthesized IGF1R polypeptides at the endoplasmic reticulum, inducing their ubiquitination and proteasome-mediated degradation. In the absence of AIRAPL, the lack of this regulatory mechanism increases IGF1R steady-state levels, promoting insulin/IGF-1 signaling deregulation.
Numerous studies have evaluated IGF1R as a potential therapeutic target for solid tumors, but its clinical relevance in myeloid malignancies has not been similarly addressed.Citation7 In this regard, we have demonstrated the critical involvement of IGF-1 signaling deregulation in MPN, as both genetic and pharmacological inhibition of IGF1R prevented MPN-related alterations in AIRAPL-deficient mice as well as in mice carrying the Jak2V617F mutation. Moreover, our in vitro and in vivo studies have revealed that AIRAPL expression is suppressed in human MPN, also supporting the efficacy of IGF1R inhibitory strategies in human MPN. These evidences constitute an important preclinical evidence of the potential of IGF1R inhibitors for the treatment of these neoplasias, alone or in combination with JAK2 inhibitors. Furthermore, we have identified the mechanism responsible for AIRAPL suppression in MPN, which is mediated by the microRNA miR-125a-3p, thereby leading to the discovery of new prognostic and therapeutic markers for these myeloid malignancies.
Globally, our work uncovers AIRAPL biological function as a novel regulator of the somatotropic axis involved in the control of myeloid hematopoiesis, which provides new insights on the molecular relationships of aging and cancer in the hematological system. This study also illustrates that alterations in evolutionarily conserved regulatory pathways, such as the IIS, are common hallmarks of both processes. Although further work should address the precise nature of the molecular signals that regulate AIRAPL function, it is tempting to speculate that its location at the ER would allow this proteostasis factor to act as a sensor of different extrinsic and intrinsic stress signals. Therefore, Zfand2b-deficient mice provide a valuable experimental system for studying MPN and also for assessing the implication of AIRAPL in other pathological conditions through IGF1R-dependent and independent mechanisms.
Finally, our study represents a clear demonstration of the oncogenic relevance of proteostasis deregulation in haematopoietic cells. Notably, most studies of the molecular mechanisms controlling HSC homeostasis have focused on the analysis of genetic and epigenetic alterations of key oncogenes and tumor suppressors, as well as on the evaluation of the signaling and transcriptional changes induced by these genomic damages. However, our finding that loss of AIRAPL causes transformation of myeloid cells provides causal evidence about the crucial role of proteostatic processes in the regulation of quiescence, proliferation and differentiation of HSCs, which finally contribute to maintain normal adult hematopoiesis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Dykstra B, et al. J Exp Med 2011; 208(13):2691–703; PMID:22110168; http://dx.doi.org/10.1084/jem.20111490
- Lopez-Otin C, et al. Cell 2013; 153(6):1194–217; PMID:23746838; http://dx.doi.org/10.1016/j.cell.2013.05.039
- Osorio FG, et al. Nat Med 2016; 22(1):91–6; PMID:26692333; http://dx.doi.org/10.1038/nm.4013
- LaFave LM, Levine RL. Nat Med 2016; 22(1):20–1; PMID:26735404; http://dx.doi.org/10.1038/nm.4028
- Yun C, et al. Proc Natl Acad Sci U S A 2008; 105(19):7094–9; PMID:18467495; http://dx.doi.org/10.1073/pnas.0707025105
- Braunstein I, et al. Mol Biol Cell 2015; 26(21):3719–27; PMID:26337389; http://dx.doi.org/10.1091/mbc.E15-02-0085
- Pollak M. Nat Rev Cancer 2008; 8(12):915–28; PMID:19029956; http://dx.doi.org/10.1038/nrc2536
