ABSTRACT
Although Ras is a potent oncogene in human tumors it has the paradoxical ability to promote Oncogene Induced Senescence (OIS). This appears to serve as a major barrier to Ras driven transformation in vivo. The signaling pathways used by Ras to promote senescence remain relatively poorly understood, but appear to invoke both the p53 and the Rb master tumor suppressors. Exactly how Ras communicates with p53 and Rb has remained something of a puzzle. NORE1A is a direct Ras effector that is frequently downregulated in human tumors. We have now found that it serves as a powerful Ras senescence effector. Moreover, we have defined signaling mechanisms that allows Ras to control both p53 and Rb post-translational modifications via the NORE1A scaffolding molecule. Indeed, NORE1A can be detected in complex with both p53 and Rb. Thus, by coupling Ras to both tumor suppressors, NORE1A forms a major component of the Ras senescence machinery and serves as the missing link between Ras and p53/Rb.
KEYWORDS:
The Ras oncoprotein may be the most potent and frequently activated oncoprotein yet detected in human cancer.Citation1 It is activated by point mutations in ~30% of all human tumors and serves as a driver for many more due to defects in its negative regulators such as NF1.Citation2 However, although activated Ras is a powerful driver of growth and transformation in many experimental systems, aberrant Ras activity in primary cells leads to growth arrest, senescence and death.Citation3 This paradoxical effect is known as oncogene induced senescence (OIS) and appears to be a major barrier preventing the development of cancer which must be overcome to permit Ras driven tumorigenesis. Indeed, many Ras tumors demonstrate high levels of senescence and the senescence levels decrease as the tumor progresses to malignancy.Citation4
While the signaling pathways utilized by Ras to promote growth are well characterized, those used by Ras to promote senescence are not well understood, but presumably they must be subverted to allow breaching of the senescence barrier. It is now evident from work by us and others that Ras interacts with a distinct set of effectors, separate from the well characterized growth promoting effectors, which promote its growth inhibitory functions. Key among these is the RASSF family of tumor suppressors which serve as Ras death effectors, binding directly to activated Ras and connecting Ras to pro-apoptotic pathways such the hippo pathway. Suppression of RASSF1A, one of the best characterized family members, impairs the ability of Ras to induce apoptosis. NORE1A (RASSF5), another major RASSF family member, shares ~50% homology to RASSF1A but is much less pro-apoptotic. Instead, it induces a powerful senescence response. Indeed, over-expression of NORE1A is as effective as over-expressing activated Ras at inducing senescence. NORE1A appears to repress the pro-transformation properties of Ras in that suppression of NORE1A by shRNA dramatically blocks both basal levels of senescence and the ability of Ras to induce senescence. This results in an enhanced transforming activity of Ras in the absence of NORE1A.Citation5
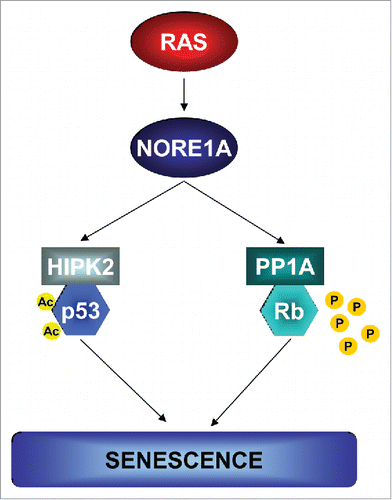
Our original work showed that NORE1A acts to link Ras to the acetylation of the p53 tumor suppressor, promoting a pro-senescence post-translational modification dependent upon the kinase HIPK2 coupling acetyltranferases PCAF and p300 to p53.Citation5 However, suppression of p53 only partially inactivated the NORE1A senescence phenotype.Citation5 Since in human cells, full senescence likely requires both p53 and Rb activation,Citation6 we went on to examine the effects of NORE1A on Rb.
Rb is regulated in large part by a phosphorylation/dephosphorylation cycle. One of the most important components regulating the dephosphorylation and activation of Rb is the phosphatase PP1A. We found that NORE1A forms an endogenous, Ras regulated complex with PP1A that scaffolds PP1A to Rb, enhancing Rb dephosphorylation, thereby activating Rb.Citation7
Thus, the potency of NORE1A to act as a Ras senescence effector stems from its ability to simultaneously activate both p53 and Rb (). In both cases, the mechanism involves Ras regulated scaffolding of a kinase or a phosphatase, respectively, to its target. Loss of NORE1A would therefore uncouple Ras from both p53 and Rb, thereby subverting the protective senescence barrier and allowing Ras pro-growth signals to predominate. In human tumors, loss of NORE1A correlates closely not only with the development of malignancy, but also with the loss of expression of senescence markers. NORE1A suppression may therefore be a key element in the ability of Ras driven tumors to breach the senescence barrier and explains why Ras driven tumors are frequently associated with reduced NORE1A expression. Thus, NORE1A is a double barreled senescence effector for Ras with a complex mode of action.
Figure 1. Mechanisms of Ras-induced senescence. Ras utilizes its death effector NORE1A, to activate both the p53 and Rb tumor suppressor pathways to induce senescence. Ras promotes the acetylation of p53, via HIPK2, to activate its senescent properties while simultaneously activating Rb through PP1A-mediated dephosphorylation. Loss of NORE1A would therefore bypass both these senescence pathways, allowing the transforming properties of Ras to predominate. Ac, acetylated lysine; P, phosphorylation.
These may not be the only mechanisms by which NORE1A invokes senescence. We have also found that NORE1A activates the expression of IL-6,Citation7 a pleiotropic cytokine involved not only in inflammation but also in the induction of senescence. IL-6 is secreted by multiple cell types in the tumor microenvironment and contributes to the senescence-associated secretory phenotype (SASP). SASP factors act via both autocrine and paracrine modes to either promote tumorigenesis or reinforce the senescent phenotype. IL-6 is one of the factors that reinforces the senescence process. Although we observe increasing loss of NORE1A expression in primary tumor tissue as they as progress to malignancy, we have observed an elevated level of NORE1A expression in the stroma surrounding the tumors. Thus, upregulation of NORE1A may be contributing to the SASP in stromal cells while down-regulation in the tumor cells promotes senescence resistance. Future work will determine whether NORE1A contributes to additional senescence pathways and how NORE1A contributes to SASP. It may also determine if NORE1A has physiological functions that are independent of Ras, like its shorter splice variant NORE1B.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Malumbres M, Barbacid M. Nat Rev Cancer 2003; 3:459-65. PMID:12778136; http://dx.doi.org/10.1038/nrc1097
- Cox AD, et al. Nat Rev Drug Discov 2014; 13:828-51. PMID:25323927; http://dx.doi.org/10.1038/nrd4389
- Serrano M, et al. Cell 1997; 88:593-602. PMID:9054499; http://dx.doi.org/10.1016/S0092-8674(00)81902-9
- Collado M, Serrano M. Nat Rev Cancer 2010; 10:51-7. PMID:20029423; http://dx.doi.org/10.1038/nrc2772
- Donninger H, et al. J Cell Biol 2015; 208:777-89. PMID:25778922; http://dx.doi.org/10.1083/jcb.201408087
- Kuilman T, et al. Genes Dev 2010; 24:2463-79. PMID:21078816; http://dx.doi.org/10.1101/gad.1971610
- Barnoud T, et al. J Biol Chem 2016 Feb 5; 291(6):3114-3123. PMID:26677227.
