Cytokinesis is the physical division of one cell into two daughter cells that occurs at the end of the cell cycle.Citation1 Cytokinesis is thought to be fundamentally similar in all metazoan divisions-that is, driven by constriction of an equatorial actomyosin contractile ring positioned and controlled by signals from the mitotic spindle. However, there are two main types of animal cell division: symmetric and asymmetric. Symmetric divisions produce daughter cells with equal cytoplasmic and cortical components, and thus the same cell size and fate. In contrast, many embryonic and stem cell divisions are asymmetric, resulting in daughter cells of differing cell size and/or cell fate. It is unknown if cytokinesis is differentially regulated in symmetric and/or asymmetrically dividing cells.
During asymmetric cell divisions, the PAR proteins (PARtitioning defective) control the unequal inheritance of key cell fate determinants.Citation2 The C. elegans single-cell zygote has been a seminal system for understanding the molecular regulation of anterior-posterior (A-P) polarity. In this system, fertilization triggers polarity establishment and the formation of opposing anterior PAR (aPAR) and posterior PAR (pPAR) domains within the zygote. Anterior-directed actomyosin-based cortical flow leads to the enrichment of cortical aPARs (myosin-II, PAR-3, PAR-6, PKC-3, and CDC-42) on the anterior cell cortex. At the same time, the cortical pPAR protein PAR-2 localizes to the posterior cortex where it excludes the aPARs. The aPARs and pPARs remain on opposing sides of the cell cortex, a state maintained via mutual inhibition up to and during cytokinesis.Citation2
The core cortical PAR proteins were previously thought to control cytokinesis indirectly by positioning the mitotic spindle and spindle-associated signals, which in turn control the contractile ring during cytokinesis. However, experiments in asymmetrically dividing Drosophila neuroblasts revealed a polarity-dependent contractile ring can form when the spindle is perturbed.Citation3
We sought to test if the polarity machinery plays a direct role in contractile ring constriction in the C. elegans zygote. We utilized fast-acting temperature sensitive mutants of two core contractile ring components: the motor myosin-IINMY-2, and the f-actin nucleator diaphanous forminCYK-1. Both of these alleles show a full loss-of-function phenotype at restrictive temperature and can be inactivated within ≤17 seconds using a fluidic device.Citation4 Whereas myosin-IINMY-2 is required for both polarity establishment and cytokinesis, forminCYK-1 only plays a role in cytokinesis. We took advantage of the tunability of these mutant alleles and examined cytokinesis at semi-permissive temperatures, where both myosin-IInmy-2(ts) and formincyk-1(ts) single mutants successfully complete cytokinesis. Interestingly, we found 40% of myosin-IInmy-2; formincyk-1(ts) double mutants failed in cytokinesis when upshifted to semi-permissive temperature, but when upshifted early in the cell cycle during polarity establishment, not during mitosis or cytokinesis directly. This suggested that the early role of myosin-II in cell polarity is required later in the cell cycle for cytokinesis.
To test this more directly we depleted the cortical PAR polarity proteins in both myosin-IInmy-2(ts) and formincyk-1(ts) mutants. Indeed, we found depletion of either the aPARs (CDC-42, PAR-3, PAR-6, or PKC-3) or the pPAR PAR-2 led to cytokinesis failure in formincyk-1(ts), but not myosin-IInmy-2(ts) mutants. This suggests that polarized cells are more resistant to loss of f-actin at the contractile ring. Moreover, we found that depletion of the cortical aPAR and pPAR proteins both lead to reduced f-actin accumulation in the contractile ring, suggesting this is a key downstream effect of PAR-mediated polarity during cytokinesis.
To link the effect of polarity on contractile ring f-actin and cytokinesis, we investigated two actomyosin cross-linking proteins that have been implicated in cytokinesis in multiple systems, septin and anillin.Citation5 We found both septin and anillin are enriched in the cell anterior throughout polarity establishment and cytokinesis and this localization is dependent upon the PAR proteins. Depletion of the PAR proteins also led to an increase in the levels of both septin and anillin in the contractile ring. Surprisingly, we found that depletion of either septin or anillin increased the levels of f-actin at the contractile ring. Finally, depletion of either septin or anillin suppressed the cytokinesis failure in formincyk-1(ts) mutants (but not myosin-IInmy-2(ts) mutants), even when polarity was co-disrupted. This suggests that in the asymmetrically dividing C. elegans zygote, septin and anillin are negative regulators of contractile ring f-actin, an unexpected result for proteins previously thought to play a positive role in contractile ring assembly during cytokinesis. Consistent with a negative role, both septin and anillin are dispensable for contractile ring assembly and constriction in many cell types, including C. elegans embryos,Citation5 budding and fission yeast, some Drosophila cell types, and many human cell linesCitation6 (for more references, see discussion inCitation7).
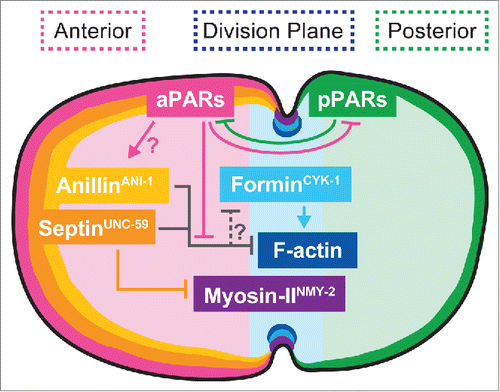
We propose a model in which PAR-dependent sequestration of septin and anillin is required to prevent over-accumulation of these inhibitors of f-actin in the contractile ring (). This unanticipated finding highlights the dramatically different roles that these proteins may play depending on the organism and/or cell-type. Our model also highlights that crosslinking forces in the contractile ring and in the cell cortex, as well as linkages between these structures, must be balanced to allow productive constriction. Further investigation into the intersection between cellular polarity and cytokinesis will be required to determine how these two seemingly independent cellular events interact.
Figure 1. Proposed spatial and genetic model for the molecular regulation of cytokinesis during asymmetric cell division. The aPAR and pPAR polarity proteins maintain cortical asymmetry and promote the anterior retention of the f-actin inhibitors septin and anillin, allowing normal f-actin assembly at the division plane.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Green RA, Paluch E, Oegema K. Cytokinesis in animal cells. Annu Rev Cell Dev Biol 2012; 28:29-58; PMID:22804577; http://dx.doi.org/10.1146/annurev-cellbio-101011-155718
- Motegi F, Seydoux G. The PAR network: redundancy and robustness in a symmetry-breaking system. Philos Trans R Soc Lond B Biol Sci 2013; 368:20130010; PMID:24062581; http://dx.doi.org/10.1098/rstb.2013.0010
- Cabernard C, Prehoda KE, Doe CQ. A spindle-independent cleavage furrow positioning pathway. Nature 2010; 467:91-4; PMID:20811457; http://dx.doi.org/10.1038/nature09334
- Davies T, Jordan SN, Chand V, Sees JA, Laband K, Carvalho AX, Shirasu-Hiza M, Kovar DR, Dumont J, Canman JC. High-resolution temporal analysis reveals a functional timeline for the molecular regulation of cytokinesis. Dev Cell 2014; 30:209-23; PMID:25073157; http://dx.doi.org/10.1016/j.devcel.2014.05.009
- Maddox AS, Lewellyn L, Desai A, Oegema K. Anillin and the septins promote asymmetric ingression of the cytokinetic furrow. Dev Cell 2007; 12:827-35; PMID:17488632; http://dx.doi.org/10.1016/j.devcel.2007.02.018
- Menon MB, Gaestel M. Sep(t)arate or not - how some cells take septin-independent routes through cytokinesis. J Cell Sci 2015; 128:1877-86; PMID:25690008; http://dx.doi.org/10.1242/jcs.164830
- Jordan SN, Davies T, Zhuravlev Y, Dumont J, Shirasu-Hiza M, Canman JC. Cortical PAR polarity proteins promote robust cytokinesis during asymmetric cell division. J Cell Biol 2016; 212:39-49; PMID:26728855; http://dx.doi.org/10.1083/jcb.201510063
