Fine regulation of differentiation and expansion of adult stem cells is required for the maintenance of tissue homeostasis. Deregulation toward expansion of adult stem cells is a common characteristic of poorly differentiated cancer and cancer stem cells. In this context, YAP (Yes associated protein) has gained considerable research attention as a critical mediator involved in promoting expansion and inhibiting differentiation of adult stem cells and cancer stem cells. YAP is often highly expressed in stem/progenitor compartments and its overexpression leads to uncontrolled expansion of stem/progenitor cells in various epithelial tissues, such as lung, skin, and intestine.Citation1 Highlighting its importance in cancer, YAP is frequently overexpressed in human tumor cells. In addition, it has been shown that TAZ, a paralog of YAP, is critical for generation of breast cancer stem cells.Citation2 Despite YAP's powerful ability to expand adult stem cells and cancer stem cells, the downstream target genes required for its function are not fully characterized.
In our recent study,Citation3 we used mammary epithelial cells and breast cancer cells as models to delineate downstream mechanisms underlying the stem-cell-property–inducing function of YAP. Using MCF-10A immortalized mammary epithelial cells, we found that YAP overexpression upregulates mammary stem cell (MaSC) signature genes, including interleukin 6 (IL6), which we found to be a novel, critical target of YAP in the induction of MaSC-like properties. We further found that the transcription factor SRF (serum response factor) binds YAP, recruiting it to target genes that are specifically involved in endowing mammary epithelial cells with MaSC-like properties. Extending these insights to cancer, we found that SRF, YAP/TAZ and IL6 are all overexpressed in basal-like and triple-negative breast cancer cells. These cell types are more mammary progenitor-like compared with estrogen-receptor–positive luminal-type breast cancer cells, which resemble terminally differentiated ductal cells in the mammary gland. Moreover, having found that SRF, which is critical for YAP-induced MaSC-like properties, is expressed at a low level in luminal-type breast cancer, we hypothesized that YAP may be more efficient in promoting IL6 expression and subsequent cancer stemness in basal-like breast cancer compared with luminal-type breast cancer. Indeed, we demonstrated that YAP promotes IL6 expression and generation of cancer stem cells and is correlated with poor relapse-free survival specifically in basal-like breast cancer, and not in luminal-type breast cancer.
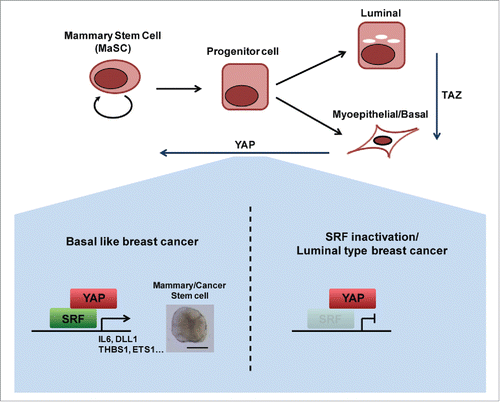
Initial in vivo support for our findings is provided by a recent study showing that either overexpression of YAP or deregulation of the Hippo pathway—the emerging YAP-restraining tumor-suppressor pathway—leads to defects in terminal differentiation of mammary epithelial cells in a mouse model.Citation4 A study by Kupperwasser and colleagues revealed that TAZ activation mediates a lineage switch from luminal fate to myoepithelial/basal fate, both in vitro and in vivo.Citation5 Our studies on MCF-10A cells take this process a step further, showing that YAP overexpression in these cells, which already possess myoepithelial/basal traits, confers MaSC-like properties. Thus, the current understanding is that YAP and TAZ are involved at various stages in the mammary epithelial cell differentiation hierarchy (). Future in vivo studies should confirm the involvement of SRF and IL6 in stem/progenitor cell expansion and cancer upon Hippo pathway deregulation or YAP activation, and refine the common and distinct roles of YAP and TAZ along the hierarchy of mammary epithelial cell differentiation.
Figure 1. SRF and IL6 are essential for YAP induction of MaSC-like properties in basal-like breast cancer. TAZ is involved in lineage switching from luminal to basal/myoepithelial cell type. YAP is involved in endowing mammary stem cell properties. YAP mediates this function by binding SRF to induce the expression of mammary stem cell signature genes specifically in basal-like breast cancer, but not in luminal-type breast cancer.
We were surprised to discover that TEAD, the transcription factor that mediates a majority of YAP's oncogenic properties, is dispensable for YAP promotion of MaSC-like properties, finding instead that YAP binds SRF as an alternative transcription factor to produce this outcome. Although our study demonstrated that the SRF-YAP transcriptional complex was independent of TEAD and conventional SRF transcription complexes involving MRTF (myocardin-related transcription factor), it should be noted that SRF-YAP regulates a specific subset of YAP targets involved in promoting MaSC-like properties. Thus, TEAD still likely remains a major mediator of YAP's oncogenic activity. Curiously, several recent studies have reported that multiple transcriptional regulators, including TEAD, SRF, MRTF and YAP, may act in concert. TEAD-YAP targets, such as CTGF (connective tissue growth factor) and CYR61 (cysteine rich angiogenic inducer 61), are also known SRF-MRTF targets. A recent ChIP-seq analysis revealed that SRF and MRTF proteins are enriched at endogenous TEAD-binding DNA motifs in intact cells, indicating possible involvement of SRF-MRTF in TEAD-mediated transcription.Citation6 It has also been found that MRTF and YAP physically interact and function together to activate target genes downstream of G protein-coupled receptors.Citation7 Importantly, all four of these transcription regulators are activated by a common set of extracellular stimuli, including RhoA activation, cytoskeletal assembly, and serum. Taken together with recent findings, this suggests the intriguing hypothesis that these transcription factors act together to exert a coordinated transcriptional response to various external stimuli, a concept that warrants intense future study.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Piccolo S, et al. Physiol Rev 2014; 94:1287-312; PMID:25287865; http://dx.doi.org/10.1152/physrev.00005.2014
- Cordenonsi M, et al. Cell 2011; 147:759-72; PMID:22078877; http://dx.doi.org/10.1016/j.cell.2011.09.048
- Kim T, et al. Nat Commun 2015; 6; PMID:26671411; http://dx.doi.org/10.1038/ncomms10186
- Chen Q, et al. Genes Dev 2014; 28:432-7; PMID:24589775; http://dx.doi.org/10.1101/gad.233676.113
- Skibinski A, et al. Cell Rep 2014; 6:1059-72; PMID:24613358; http://dx.doi.org/10.1016/j.celrep.2014.02.038
- Esnault C, et al. Genes Dev 2014; PMID:24732378; http://dx.doi.org/10.1101/gad.239327.114
- Yu O, et al. Mol Cell Biol 2015; PMID:26459764; http://dx.doi.org/10.1128/mcb.00772-15
