ABSTRACT
While primary open-angle glaucoma (POAG) is a leading cause of blindness worldwide, it still does not have a clear mechanism that can explain all clinical cases of the disease. Elevated IOP is associated with increased accumulation of extracellular matrix (ECM) proteins in the trabecular meshwork (TM) that prevents normal outflow of aqueous humor (AH) and has damaging effects on the fine mesh-like lamina cribrosa (LC) through which the optic nerve fibers pass. Applying a pathway analysis algorithm, we discovered that an elevated level of TGFβ observed in glaucoma-affected tissues could lead to pro-fibrotic pathway activation in TM and in LC. In turn, activated pro-fibrotic pathways lead to ECM remodeling in TM and LC, making TM less efficient in AH drainage and making LC more susceptible to damage from elevated IOP via ECM transformation in LC. We propose pathway targets for potential therapeutic interventions to delay or avoid fibrosis initiation in TM and LC tissues.
Introduction
Glaucoma, one of the major ocular diseases, is characterized by neuropathy involving progressive loss of retinal ganglion cells, cupping and atrophy of the optic nerve head, and associated visual field loss.Citation1,2 The most common form, primary open-angle (POAG) glaucoma, is characterized by the free aqueous humor (AH) outflow between the iris and cornea in an open anterior chamber angle, which cannot drain through the trabecular meshwork (TM) due to clogged channels.Citation3
Although the nature of POAG is multi-factorial and involves complex molecular networks, including genetic and environmental factors,Citation2,4 previous studies have identified increased TGFβ levels in AH and extracellular matrix (ECM) of TM is one of the major molecular signatures of glaucoma, and specifically POAG.Citation5,6 TGFβ-induced expression of tissue transglutaminase (tTgase)Citation7 irreversibly cross-links ECM proteins and may ultimately resist the AH outflow.Citation6,7 Moreover, TGFβ causes an increase in expression of plasminogen activator inhibitor (PAI-1) and prevents activation of a number of matrix metalloproteinases via tissue plasminogen activator (tPA), contributing to the excess ECM deposition and inhibition of TM cell proliferation.Citation8 Additionally, its interaction with the connective tissue growth factor (CTGF) may modulate the cellular configuration in the juxtacanalicular TM region by driving cells into a contractile myofibroblastic phenotype, further contributing to overall tissue stiffening.Citation9 This increased ECM accumulation and hardening subsequently leads to gradual increase in the AH outflow resistanceCitation10,11 and intraocular pressure (IOP).Citation10,12 Abnormally elevated IOP applies pressure on the optic nerve head and obstructs axoplasmic flow within the retinal ganglion cell (RGC) axons at the lamina cribrosa (LC), alters optic nerve microcirculation and is implicated in changes in the laminar glial and connective tissue.Citation13
Although TFGβ antagonists, and inhibitors of its downstream targets, demonstrated a significant protective effect in cell-lines and mouse models,Citation14,15 their effectiveness in lowering IOP have not been clinically validated, and the exact mechanism underlying increase in resistance to AH outflow through the trabecular pathway is largely unknown. Therefore, a better understanding of the molecular pathways associated with the response of ocular connective tissue to the effect of the IOP may lead to detection of other potential targets for glaucoma therapy and eventually an improved outcome for patients.
To this end, we used a novel software suite, AMD Medicine, for quantitative and qualitative analysis of intracellular signaling pathway activation (SPA)Citation16,17 based on the gene expression profiles from human TM or LC cultured cells and from human samples of TM or LC with or without POAG, derived from well annotated publicly available datasets (GSE4316, GSE27276, GSE45570, GSE13534, GSE758, E-MEXP-3440, GSE2378, GSE2705). The intracellular SPA analysis is a universal method, which may be used to analyze any physiological, stress, malignancyCitation18 or other perturbed conditions at the molecular level. In contrast to other existing techniques for aggregation and generalization of the gene expression data for individual samples, our method distinguishes the positive/activator and negative/repressor role of every gene product in each of the distinct signaling pathways analyzed and determines its pathway activation score (PAS).Citation16 Applying our pathway analysis algorithm, we explored signaling pathway activation profiles as a result of pathological conditions and we have discovered that elevated levels of TGFβ in glaucoma-affected tissues are strongly associated with the activation of pro-fibrotic pathways in TM and LC. The elevation in fibrosis-associated signaling may lead to enhanced ECM remodeling, making TM less efficient in humor drainage and causing LC to be more susceptible to damage from elevated IOP via increased tissue stiffness and ECM crosslinking.
Although our findings are exploratory, and are therefore useful primarily for the generation of new hypotheses, given the universal applicability of our platform as a potent in-silico drug screening and efficacy prediction tool,Citation16,17,19 our work may provide a roadmap for potential therapeutic interventions to delay or avoid fibrosis initiation in TM and LC tissues.
Results
Differential signaling pathway activation in trabecula meshwork tissue of POAG donors
In order to discover the signaling pathway profiles associated with the onset of glaucoma, glaucoma-related symptoms and secondary effects due to progression of the disease, we performed an in silico pathway activation analysis based on the publicly available datasets (obtained from NCBI GEO and ArrayExpress repositories) on gene-expression studies involving TM and LC. To study the general pathway activation drift in TM of the POAG donors, we applied our pathway analysis algorithm on the transcriptomic data obtained from all known POAG TM datasets, namely, GSE27276 (13 controls and 15 POAG cases) and GSE4316 (3 controls and 2 POAG cases).
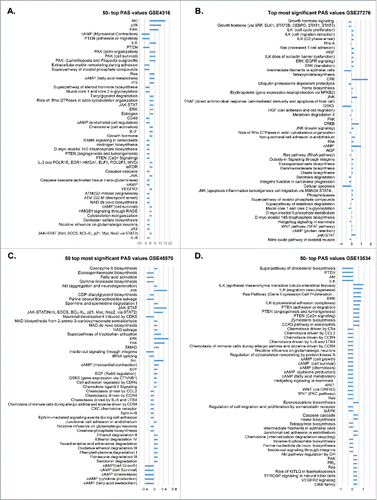
The GSE4316 data set contained only 2 POAG samples, making it impossible to estimate the statistical significance of the obtained results. For this reason, the 50 most dysregulated pathways in the TM of these 2 POAG samples compared to controls have been selected based on their corresponding PAS values (). Since dataset GSE27276 contained a sufficient number of POAG samples to estimate the p-value for each dysregulated pathway, the top 50 differentially activated signaling pathways have been selected based on their statistical significance (p < 0.00002), instead of their corresponding PAS values (). Despite different criteria used for pathway selection, a substantial overlap in most dysregulated pathways has been seen between the 2 data sets analyzed. Notably, all 50 most dysregulated pathways from the GSE4316 dataset, had also been discovered as differentially activated compared to controls in the analysis of the GSE27276 data set, with p-value <0.05. Interestingly, multiple pro-survival pathways associated with fibrogenesis in different human organs (such as AKT, PAK, p38, ERK, JNK, CREB, cAMP and JAK-STAT) were detected as unregulated in POAG tissue from both datasets ().
Figure 1. Signaling pathway activation profiles in glaucoma. Pathway activation strength (PAS) values were calculated by processing transcriptomic data obtained in human trabecular meshwork samples (datasets GSE4316 (A) and GSE27276 (B)) or lamina cribrosa samples (data sets GSE45570 (C) and GSE13534 (D)) using the AMD Medicine software suite. The fifty most dysregulated pathways compared to normal controls are shown. Blue bars represent PAS averages for each pathway denoting the degree of up regulation or down regulation. PAS presented on this figure passed the following filters PAS<‐1.5 and PAS>1.5 in all 4 datasets.
Signaling pathways differentially activated in lamina cribrosa of the POAG donors
To analyze pathway activation drift as a result of pathophysiological changes in lamina cribrosa during glaucoma progression, we analyzed data set GSE45570 containing samples of optic nerve head (ONH) which includes lamina cribrosa tissues (mixture of ECM, astrocytes, neurons fibers and glial fibrillary acid protein (GFAP) negative lamina cribrosa cells) and dataset GSE13534 containing purified GFAP LC cells respectively from POAG donors and normal control. Given the difficulty of obtaining gene expression data of well annotated, clinically-relevant LC specimens, these were the only POAG data sets currently available. For visualization of pathway activation drift in glaucoma-affected LC, we selected the 50 most significant pathways from the GSE45570 dataset with p-value cutoff 0.05 and the 50 most (judged by PAS value) dysregulated pathways from the GSE13534 data set, since it contains only 2 samples. We found that pathways like JNK, JAK-STAT, PAK, ERK, AKT were up-regulated in POAG ONH () whereas ILK, RAS, ERK, PTEN pathways related to adhesion, migration, angiogenesis and Ca2+ signaling were upregulated in POAG LC cells (). The most significant down-regulated pathways in LC cells were those including the super pathway of cholesterol biosynthesis, PTEN, AKT, Integrin-linked kinase (ILK), Hedgehog and WNT signaling ().
Effect of increased pressure on signaling pathway activation in cultured ONH astrocytes
To further elucidate the signaling changes induced by elevated pressure in LC tissue, we have analyzed a transcriptomes derived from cultured ONH astrocytes exposed to a transient increase in hydrostatic pressure (HP), dataset GSE758. Cultured astrocytes were exposed to hydrostatic pressure (60 mm Hg), compared to control astrocytes at ambient pressure (AP), over 6 hr, 24 hr and 48 hr intervals. Our analysis showed that the PAS values of 58, 21 and 40 distinct pathways, were significantly dysregulated compared to controls at 6, 24 and 48 hours respectively (Table S1). To restrict out analysis to pathways that most probably have been dysregulated as a result of increased pressure, we have selected signaling axes that were significantly up- or downregulated at all 3 time intervals. Pathways like the Wnt main pathway, Wnt-PKC, Wnt/CREB3, p38, ILK related to cytoskeletal adhesion complexes, growth hormone and glucocorticoid receptor (inflammatory cytokines) were significantly altered in ONH astrocytes under raised HP conditions, when compared to control cells (). The ILK pathway related to cytoskeletal adhesion was down-regulated 3-fold after 6 hr of exposure, and then up-regulated after 24 hr and finally down-regulated after 48 hours (). Although ILK signaling was not previously shown in glaucoma, elevated ILK signaling was associated with increased EMC remodeling in response to mechanical stiffness in lung.Citation20 Therefore, during raised IOP conditions in glaucoma, increased ILK levels may take part in microtubule and microfilament re-arrangement upon cytoskeletal remodeling of the LC cells. Interestingly, blocking ILK in TM cells results in reduced cell spreading, actin polymerization, and the localization of talin and ILK in focal adhesions (FAs). This suggests that in POAG ONH astrocytes, changes in ILK activity induced by the elevated IOP levels, may affect actin cytoskeleton organization and contractility in the TM.Citation21
Figure 2. Effect of increased pressure on signaling pathway activation in cultured lamina cribrosa cells. Pathway activation strength (PAS) values were calculated by processing and analyzing the data set GSE758 using the AMD Medicine software suite to understand the signaling pathway profile associated with elevated pressure in lamina cribrosa. Blue bars, Orange bars and Gray bars represent PAS averages and the degree of pathway up-regulation or down-regulation after 6 hour, 24 hour and 48 hour exposure to elevated pressure, respectively.
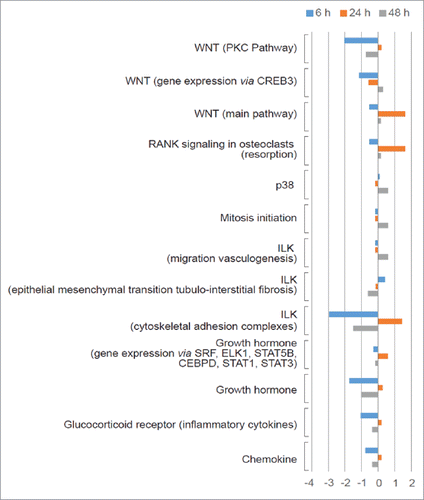
p38 is involved in the disruption of glucocorticoid receptor (GR) activity leading to a reduced GR nuclear translocation and signal transduction.Citation22 Correspondingly, an up-regulation of the p38 pathway was seen 48 hours post-exposure, which paralleled a down-regulation in the GR pathway (activated by inflammatory cytokines) (). Since glucocorticoids mediate anti-inflammatory signaling pathways, their signaling may be down-regulated to facilitate the inflammatory responses generated from optic neuropathy associated with POAG.
The Wnt main pathway is up-regulated after 24 hours of exposure but then down-regulated after 48 hours along with the Wnt/PKC and Wnt/CREB3 pathways (). Dysregulation in the Wnt pathway is known to contribute to retinal degeneration, cataract, exudative vitreoretinopathy, ocular tumor, and several congenital eye disorders.Citation23 Moreover, myocilin may act as a modulator of Wnt signaling through interactions with the Fzd family of Wnt receptors and antagonists of Wnt signaling like sFRPs and WIF-1.Citation23 Therefore, in the case of myocilin linked-POAG, elevated myocilin levels may also contribute to dysregulation of the Wnt pathway. Overexpression of the Wnt antagonist sFRP1 in the mouse eye caused rapid elevation of IOP,Citation23 suggesting that down-regulation of Wnt signaling may underlie the glaucomatous phenotype.
Signaling pathways activated in MYOC mutants
Myocilin (MYOC), a protein with cytoskeletal function, is highly expressed in TM, ciliary body and can be found in AH.Citation24,25 MYOC mutations are responsible for 3–4% of adult-onset POAG.Citation24,26 Wild type MYOC is processed and secreted into TM ECM where it interacts with fibronectin whereas mutated MYOC can accumulate in cells and can impair the function of the TM, thereby resisting AH outflow and increasing IOP.Citation24,25 More than 70 types of MYOC mutations are associated with glaucoma each having a slightly different disease phenotype.Citation26
In order to identify unique and common pathways between cells bearing wild type MYOC and mutant forms of this protein we processed data sets E-MEXP-3427, E-MEXP-3435, E-MEXP-3434 and E-MEXP-3439, representing transcriptional profiles of 4 TM cell lines each expressing 4 different MYOC mutations Q368X, R342K, D380N and K423E, and compared them to the control dataset E-MEXP-3440 of a TM cell line expressing wild type MYOC. Q368X is the most common mutant type (29% of the variants) with a mild phenotype. Although mutants R342K, D380N and K423E are much less abundant, they result in a severe clinical outcome. A Venn diagram () demonstrates that only 2 pathways (upregulation of the caspase cascade pathway and down-regulation of STAT3 pathway related to growth arrest and differentiation) were commonly dysregulated in both mutant and wild-type MYOC-bearing cells.
Figure 3. p38 signaling activation is associated with myocilin (MYOC) mutations induced glaucoma. Datasets E-MEXP-3427, E-MEXP-3435, E-MEXP-3434, E-MEXP-3439, containing gene expression profiles of 4 TM cell lines with different MYOC mutations and control data set (E-MEXP-3440) were processed and analyzed using AMD Medicine software suite. The non-intersecting blue region shows 1 pathway that was differentially activated in cell lines bearing 4 different MYOC mutants only and the non-intersecting green region shows 315 pathways that were activated in cell lines overexpressing wild type MYOC. The intersection of the blue and green circle represents pathways shared between WT MYOC and mutants with similar PAS values; the caspase cascade that is up-regulated and the STAT3 pathway that is down-regulated.
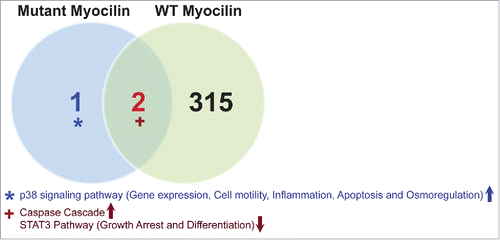
TM cells containing high levels of MYOC are highly sensitive to apoptosis.Citation27 As mentioned above, increased expression of MYOC results in loss of actin stress fibers and focal adhesions.Citation27,28 MYOC may decrease cellular adhesion to fibronectin, compromising cellular homeostasis, thereby inducing susceptibility to apoptosis.Citation28 This may explain the up-regulation on the caspase cascade revealed by our analysis.
Subsequently, a parallel decrease in STAT3 pathway related to growth and differentiation may be associated with the up-regulation of pro-apoptotic genes within the caspase cascades, leading to a pro-apoptotic fate of the cells.Citation29 Notably, the p38 signaling pathway was up-regulated in all MYOC mutants cell lines compared to the wild type control ().
TGFβ treated LC samples and glaucomatous LC samples cluster together at the pathway level
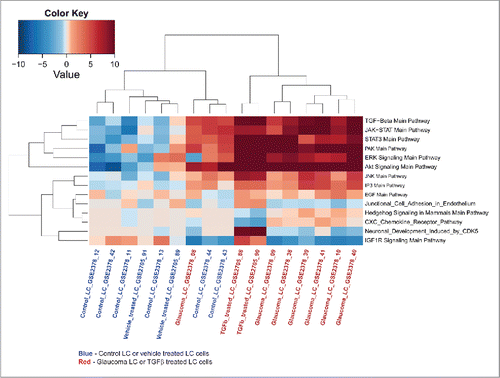
In POAG, the ECM remodeling of the LC is believed to be regulated by high levels of TGFβ and eventually lead to a fibrotic ONH.Citation30 To further delineate the intricate signaling pathways involved in the ECM remodeling of the LC, we have analyzed data sets GSE2378 and GSE2705 (containing glaucomatous astrocytes treated or untreated with TGFβ). To directly compare pathways activated in glaucomatous LC, TGFβ-treated LC and control LC, we have created the hierarchically clustered heat-map of differentially activated pathways in all 3 sample groups (). Interestingly, pathways dysregulated in TGFβ-treated cells, cluster together with pathways disturbed in glaucomatous LC, but not with the normal controls (). Notably, pro-survival pathways up-regulated in TGFβ-treated and glaucomatous LC (such as JAK-STAT, PAK, JNK and AKT), are highly associated with pro-fibrotic processes orchestrated by TGFβ,Citation31,32 further supporting the idea that TGFβ induced fibrogenesis is an integral part of the POAG development.
Figure 4. TGFβ treated LC cells and glaucoma affected LC cells cluster together at the pathway level. Datasets GSE2378 and GSE2705 (containing glaucomatous astrocytes treated or untreated with TGFβ) were processed and analyzed using the AMD Medicine software suite. To directly compare pathways activated in glaucomatous LC, TGFβ-treated LC and control LC, we have created the hierarchically clustered heatmap of differentially activated pathways in all 3 sample cohorts. Red boxes represent pathway up-regulation and blue boxes represent pathway down-regulation. PAS values generated for in-vitro TGFβ treated human LC cells substantially correlate with PAS values obtained for glaucomatous LC cells and numerous pathways dysregulated in these 2 cohorts cluster together, but not with the normal controls, suggesting that TGFβ induced fibrogenesis is an integral part of glaucoma development.
Discussion
Although POAG is a leading cause of blindness worldwide, the molecular mechanisms underlying its initiation, maintenance and progression are not yet fully understood and remain to be elucidated. Here we applied AMD Medicine, a new bioinformatics software suite, for qualitative analysis of intracellular signaling pathway activation using transcriptomic data, to assess the network of molecular signaling associated with the POAG phenotype.
It is well documented that AH outflow blockage in glaucomatous eyes results from the resistance created by changes in the quality and amount of the ECM in the juxtacanalicular region of the TMCitation33 (). These changes are believed to be highly associated with overexpression of TGFβ, which was reported to orchestrate the pro-fibrotic signaling that may affect the TM ECM turnover.Citation33 TGFβ facilitates net matrix deposition by over-production of fibronectin (FN), collagen I and IV and inhibitors of ECM proteases, such as plasminogen activator inhibitor-1 (PAI-1) and tissue inhibitors of matrix metalloproteinases (TIMPs) that degrade matrix components.Citation34,35 Subsequently, abrupt changes in ECM composition may lead to disrupted cell-cell signaling and non-specific interactions, resulting in apoptotic cell death.Citation35
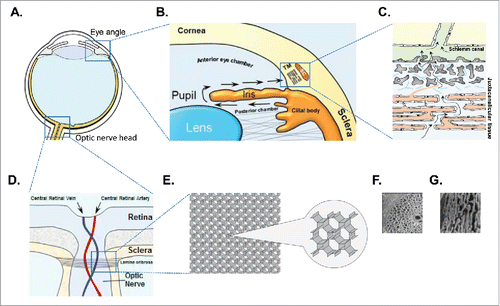
Figure 5. Trabecular meshwork and lamina cribrosa are 2 main players in glaucoma progression. Model of eye tissues associated with glaucoma pathophysiology. (A) Diagram of eye tissues involved in glaucoma pathogenesis – 2 blue rectangles: eye angle containing trabecular meshwork (TM) and optic nerve head containing lamina cribrosa (LC) (B). Eye angle containing trabecular meshwork located between the cornea and the iris. Aqueous humor (AH) is produced by the ciliary body in the posterior chamber, flows into the anterior chamber and is finally drained into the Schlemm's canal (SC) via TM. Arrows show direction of AH movement. Blue rectangle corresponds to the larger trabecular meshwork insert. (C) Trabecular meshwork insert showing AH flow (arrows) though juxtacanalicular tissue into Schlemm canel. AH outflow blockage due to clogging of TM results in elevated IOP. (D) Optic nerve head containing axons of RGC and lamina cribrosa structure. (E) Insert showing fine morphology of collagen fibers of lamina cribrosa. Lamina cribrosa ECM proper construction is vitally important for LC function. Misalignment of collagen fibers due to ECM rearrangement results in loss of mechanical resistance of LC. (F) SEM of trabecular meshwork (re-print from Citation73 with publisher permission). (G) SEM of lamina cribrosa (re-print from Citation74 with publisher permission).
Our pathway activation analysis has demonstrated a significant AKT pathway up-regulation in POAG TM samples compared to normal controls. The AKT signaling main pathway is an important regulator of cell survival in response to growth factors and other extracellular stimuliCitation36 and, when activated, exerts anti-apoptotic activity by preventing the release of cytochrome c from mitochondria.Citation37 Since glaucomatous pathophysiology involves decrease in TM cellularity due to apoptosis, RGC death from the optic neuropathy and ECM remodeling,Citation38,39 AKT activation might play a protective role against decrease in TM cellularity. Correspondingly, the substantial down-regulation of the PTEN main pathway (a negative AKT regulator) and up-regulation of the PAK main pathway (PAK acts as a scaffold to facilitate AKT stimulation by PDK1 and aids in recruitment of AKT to the membraneCitation40), seen in POAG samples, may further support these suggestions.
In addition to AKT, our analysis revealed up-regulation in other pro-survival signaling pathways in POAG TM, such as the p38 signaling main pathway, extracellular receptor kinases (ERK) and c-jun N-terminal kinases (JNK). p38 function is neuroprotective and its up-regulation facilitates RGC survival after ischemia or reperfusion injury.Citation41 Moreover, it has been reported that ERK, p38 and JNK signal transduction pathways are relatively unresponsive in glaucomatous TM cells as compared to normal cells, suggesting their constitutive signaling activity in POAG.Citation42 Additionally, through silencing of the pro-apoptotic gene BAD, and induction of the anti-apoptotic gene BCL-2,Citation43 MAPK and AKT pathways (which were up-regulated in ONH) promote neuronal survival by rescuing RGC from death occurring from optic nerve injury.Citation43,44 Interestingly, the p38 pathway related to cell motility, inflammation, apoptosis and osmoregulation was up-regulated in the cell lines over-expressing mutated MYOC. Because the MYOC expression pattern is complex, its biological function in normal and glaucomatous eyes has been difficult to elucidate. However, data indicate that POAG caused by mutations in MYOC may result primarily from protein misfolding or improper protein traffickingCitation45 and its expression is also induced by TGFβ.Citation46 Additionally, MYOC is associated with the downregulation of RhoA,Citation27 which also can facilitate phosphorylation of p38 and activation of MAPK related pathways.Citation47
The ILK signaling main pathway, which facilitates the increase in matrix metalloproteinases (MMP-2),Citation48 was also up-regulated in POAG samples compared to normal controls. Moreover, ILK pathway related to migration and vasculogenesis was significantly up-regulated in ONH astrocytes after 48 hr exposure to HP. Interestingly, in glaucomatous conditions, TM cells sense mechanical stretching and respond by increasing levels of MMP and tissue inhibitor of matrix metalloproteinase (TIMP) through integrin–ECM interactions to reverse outflow resistance and re-establish IOP homeostasis.Citation48 Therefore, it is possible that ILK protects against mechanical stretching of the ocular tissue from AH outflow blockage. Notably, several studies have shown that ILK is also involved in the activation of ERK and p38 MAPK signaling during the development of hepatic and pulmonary fibrosis,Citation49,50 suggesting that similar signal transduction cascades may also occur in POAG.
The MAPK/ERK signaling cascade is a major pathway controlling cellular processes associated with fibrogenesis, including growth, proliferation, and survival,Citation51,52 whereas PAK/P38 signaling plays a key role in pro-fibrogenic epithelial–mesenchymal transition (EMT),Citation53,54 and was shown to be up-regulated during organ fibrogenesis.Citation55,56 Moreover, ERK, p38 MAPK and ILKCitation57 pathways are principally related to TGFβ signaling,Citation58 which plays a central role in fibrotic disorders by inducing multiple pro-fibrogenic and immunosuppressive effects in various distinct organs including the eye.Citation59 In glaucoma, TGFβ-induced fibrotic processes are known to orchestrate the build-up of ECM materials in the TM at the anterior of the eye, and in the LC at the ONH.Citation60 For example, GFAP-negative LC cells constitutively release TGFβ-induced pro-fibrotic factors, such as connective tissue growth factor (CTGF) and platelet-derived growth factor-alpha (PDFG-alpha).Citation61 Moreover, TGFβ induces expression and release of collagen type IA1, α-smooth muscle actin (αSMA), and alters cell-ECM interaction rigidity in POAG LC cells.Citation30,62 Notably, we found that various pro-survival pathways highly associated with pro-fibrotic processes such as the AKT Signaling Main Pathway, CXC Chemokine Receptor Pathway, EGF Main Pathway, ERK Signaling Main Pathway, Hedgehog Signaling in Mammals Main Pathway, IGF1R Signaling Main Pathway, IP3 Main Pathway, JAK-STAT Main Pathway and JNK Main Pathway, were up-regulated in TGFβ-treated glaucomatous astrocytes and glaucomatous LC samples. Although these data support previous observations that TGFβ-induced fibrogenesis is an integral part of the POAG,Citation63 these TGFβ-induced pro-survival pathways may have a neuroprotective role against ECM remodeling and/or IOP stress-related apoptosis. These pathways may also facilitate progenitor cell proliferation and astrocyte differentiation to cope up with the damage from fibrosis.Citation64
In contrast, the glycogen synthase kinase 3 (GSK3) pathway was down-regulated in POAG TM. Down-regulation of GSK3 has been shown to induce expression of active β-catenin and to suppress levels of certain ECM proteins, even under stimulatory effects of TGFβ.Citation65 Consequently, down-regulation of GSK might play a role in activation of canonical WNT signaling by statins (HMG-CoA reductase inhibitors) and facilitate AH outflow to re-establish IOP homeostasis under glaucomatous conditions.Citation65 Interestingly, emerging evidence has shown that the cross-talk between WNT and TGFβ signaling plays important roles in TM homeostasis and IOP regulation.Citation66 While only a few genes that mediate the crosstalk between the 2 pathways are currently discovered, manipulating these mediators may provide a more effective way of restoring aqueous outflow in the TM, and possibly treating glaucoma.
Conclusion
Malfunction of 2 fine structures in the human eye (TM and LC) could lead to glaucoma since the ability to carry their function heavily depends on morphology,Citation67 which can be easily affected by pro-fibrotic ECM rearrangements (). As the main function of TM is to filter out AH, slight changes in ECM can cause clogging of the fine TM filtering system (). The main function of LC is to withstand IOP provided by precise configuration of collagen fibers, which when misaligned can cause loss of mechanical resistance and subsequent atrophy of ONH (). Only one third of people with glaucoma have normal or near normal IOP, whereas one third of patients with high IOP do not develop glaucoma,Citation68,69 which represents the main challenge in understanding glaucoma biology. While elevated IOP is a major risk factor for glaucoma, ECM remodeling of the LC as a result of pro-fibrotic processes can provide a mechanistic explanation for the glaucoma phenotype in a considerable portion of patients with normal IOP. Simultaneously, LC resistance to ECM rearrangement affecting LC ability to withstand elevated IOP could explain why some people with increased ocular pressure do not develop the glaucoma phenotype ().
Figure 6. Glaucoma pathology chart. The figure represents a graph of age vs LC resistance, on the left, and ocular pressure, on the right. LC loses its resistance with age and becomes more susceptible to damage resulting from elevated IOP. Simultaneously there is a decrease in cellularity of TM and higher resistance to AH drainage with age. These factors suggest that the risk of developing glaucoma increases with age. The glaucoma-free zones represent 2 different populations as follows: (1) population with elevated IOP which does not develop glaucoma due a fibrosis resistant LC and (2) population with glaucoma which has normal IOP but the disease develops from fibrotic changes arising directly in the LC and ONH.
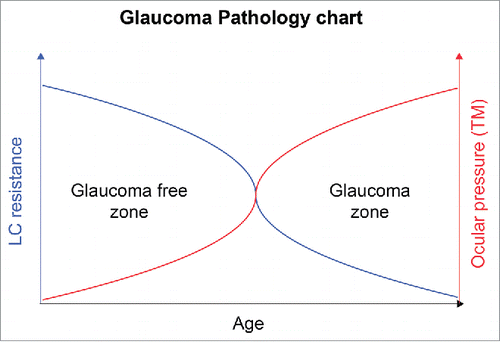
Although our results are correlations, and future confirmatory studies are warranted to validate these observations, it is tempting to speculate that until increased IOP gets counter balanced by a fibrosis resistant LC (probably thicker and smaller in diameter that can withstand elevated IOPCitation70), patients will most probably remain POAG free. However, when the LC collagen structure is affected by fibrosis and LC mechanical resistance drops below a certain threshold, it may create a favorable condition for ONH cupping and deformation (glaucoma phenotype) even with healthy TB (normal IOP) ().
While it is well known that fibrosis plays a role in the glaucoma progression and a number of therapeutic approaches have been studied in an attempt to combat fibrosis, the fibrogenesis-driving mechanisms are not yet fully understood. Therefore, computational studies, like ours, may aid in uncovering complex molecular processes underlying the fibrotic changes in glaucoma which subsequently may lead to discovery of novel attractive therapeutic targets.
Methods
Source datasets
In this study, we utilized microarray gene expression data downloaded from NCBI GEO and ArrayExpress databases in order to examine pathways that are affected by glaucoma. The following data sets have been used: GSE4316, GSE27276, GSE45570, GSE13534, GSE758, E-MEXP-3440, GSE2378, GSE2705.
Bioinformatics analysis and transcriptomic expression data pre-processing
All microarray data preprocessing steps were performed in R version 3.1.0 using packages from Bioconductor. Microarray raw data were background adjusted and quantile normalized using the corresponding R packages. Obtained gene expression values were averaged across all replicates. Heat map generation and hierarchical clustering were performed using R package gplots. Statistical tests and correlation analysis were done with the MS Excel software.
Signaling pathway analysis
Preprocessed gene expression data were loaded into AMD Medicine pathway analysis software, a proprietary suite developed by Vision Genomic, Inc. which represents a cloud based implementation of the Oncofinder algorithm,Citation16,17,19,71 optimized for AMD/Glaucoma studies. As previously described,Citation72 it enables calculation of the Pathway Activation Strength (PAS), a value which serves as a quantitative measure of differential pathway activation between the 2 states. PAS scoring is based on the expression level and the role of a particular gene in proprietary maps of 3 hundred of signaling pathways. Pathways with positive PAS values are considered up-regulated, while negative PAS values correspond to down-regulated pathways.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Supplementary Files
Download MS Excel (20.4 KB)Acknowledgments
We thank Maria Lynguzova for assistance with figure preparation and for comments that greatly improved the manuscript.
References
- Thylefors B, Negrel AD. The global impact of glaucoma. Bull World Health Organ 1994; 72(3):323-6; PMID:8062393.
- Allingham RR, Liu Y, Rhee DJ. The genetics of primary open-angle glaucoma: a review. Exp Eye Res Apr 2009; 88(4):837-44; PMID:19061886; http://dx.doi.org/10.1016/j.exer.2008.11.003
- Kroese M, Burton H. Primary open angle glaucoma. The need for a consensus case definition. J Epidemiol Community Health Sep 2003; 57(9):752-4; PMID:12933785; http://dx.doi.org/10.1136/jech.57.9.752
- Booth A, Churchill A, Anwar R, Menage M, Markham A. The genetics of primary open angle glaucoma. Br J Ophthalmol May 1997; 81(5):409-14; PMID:9227209; http://dx.doi.org/10.1136/bjo.81.5.409
- Agarwal P, Daher AM, Agarwal R. Aqueous humor TGF-beta2 levels in patients with open-angle glaucoma: A meta-analysis. Mol Vis 2015; 21:612-20; PMID:26019480.
- Gottanka J, Chan D, Eichhorn M, Lutjen-Drecoll E, Ethier CR. Effects of TGF-beta2 in perfused human eyes. Investigative ophthalmology & visual science. Jan 2004; 45(1):153-8; http://dx.doi.org/10.1167/iovs.03-0796
- Welge-Lussen U, May CA, Lutjen-Drecoll E. Induction of tissue transglutaminase in the trabecular meshwork by TGF-beta1 and TGF-beta2. Invest Ophthalmol Vis Sci. Jul 2000; 41(8):2229-38.
- Wordinger RJ, Fleenor DL, Hellberg PE, Pang IH, Tovar TO, Zode GS, Fuller JA, Clark AF. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci Mar 2007; 48(3):1191-200; PMID:17325163; http://dx.doi.org/10.1167/iovs.06-0296
- Braunger BM, Fuchshofer R, Tamm ER. The aqueous humor outflow pathways in glaucoma: A unifying concept of disease mechanisms and causative treatment. Eur J Pharm Biopharm May 7 2015; 95:173-81; PMID:25957840.
- Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci Mar 2000; 41(3):619-23.
- Toris CB, Yablonski ME, Wang YL, Camras CB. Aqueous humor dynamics in the aging human eye. Am J Ophthalmol Apr 1999; 127(4):407-12; PMID:10218693; http://dx.doi.org/10.1016/S0002-9394(98)00436-X
- Kuespert S, Junglas B, Braunger BM, Tamm ER, Fuchshofer R. The regulation of connective tissue growth factor expression influences the viability of human trabecular meshwork cells. J Cell Mol Med Feb 20 2015; 19:1010-20; PMID:25704370.
- Agarwal R, Gupta SK, Agarwal P, Saxena R, Agrawal SS. Current concepts in the pathophysiology of glaucoma. Indian J Ophthalmol Jul–Aug 2009; 57(4):257-66.
- Quigley HA, Pitha IF, Welsbie DS, Nguyen C, Steinhart MR, Nguyen TD, Pease ME, Oglesby EN, Berlinicke CA, Mitchell KL, et al. Losartan treatment protects retinal ganglion cells and alters scleral remodeling in experimental glaucoma. PloS One 2015; 10(10):e0141137; PMID:26505191; http://dx.doi.org/10.1371/journal.pone.0141137
- Meyer-Ter-Vehn T, Gebhardt S, Sebald W, Buttmann M, Grehn F, Schlunck G, Knaus P. p38 inhibitors prevent TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci Apr 2006; 47(4):1500-9; http://dx.doi.org/10.1167/iovs.05-0361
- Buzdin AA, Zhavoronkov AA, Korzinkin MB, Venkova LS, Zenin AA, Smirnov PY, Borisov NM. Oncofinder, a new method for the analysis of intracellular signaling pathway activation using transcriptomic data. Front Gen 2014; 5:55; PMID:24723936.
- Buzdin AA, Zhavoronkov AA, Korzinkin MB, Roumiantsev SA, Aliper AM, Venkova LS, Smirnov PY, Borisov NM. The OncoFinder algorithm for minimizing the errors introduced by the high-throughput methods of transcriptome analysis. Front Mol Biosci 2014; 1:8; PMID:25988149.
- Zhu Q, Izumchenko E, Aliper AM, Makarev E, Paz K, Buzdin AA, Zhavoronkov AA, Sidransky D. Pathway activation strength is a novel independent prognostic biomarker for cetuximab sensitivity in colorectal cancer patients. Hum Gen Variat. 04/02/online 2015; 2:15009-18.
- Borisov NM, Terekhanova NV, Aliper AM, Venkova LS, Smirnov PY, Roumiantsev S, Korzinkin MB, Zhavoronkov AA, Buzdin AA. Signaling pathways activation profiles make better markers of cancer than expression of individual genes. Oncotarget. Oct 30 2014; 5(20):10198-205; PMID:25415353.
- Shkumatov A, Thompson M, Choi KM, Sicard D, Baek K, Kim DH, Tschumperlin DJ, Prakash YS, Kong H. Matrix stiffness-modulated proliferation and secretory function of the airway smooth muscle cells. Am J Physiol Jun 1 2015; 308(11):L1125-1135; PMID:25724668.
- Faralli JA, Newman, JR, Sheibani, N, Dedhar S, Peters DM. Integrin-linked kinase regulates integrin signaling in human trabecular meshwork cells. Invest Ophthalmol Vis Sci Mar 2011; 52(3):1684-92.
- Wang X, Wu H, Miller AH. Interleukin 1alpha (IL-1alpha) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function. Mol Psychiat Jan 2004; 9(1):65-75; PMID:14699442.
- Kwon HS, Lee HS, Ji Y, Rubin JS, Tomarev SI. Myocilin is a modulator of Wnt signaling. Mol Cell Biol Apr 2009; 29(8):2139-54; PMID:19188438.
- Ohlmann A, Tamm ER. [The role of myocilin in the pathogenesis of primary open-angle glaucoma]. Ophthalmologe. Sep 2002; 99(9):672-7; PMID:12219254.
- Kanagavalli J, Pandaranayaka E, Krishnadas SR, Krishnaswamy S, Sundaresan P. A review of genetic and structural understanding of the role of myocilin in primary open angle glaucoma. Indian J Ophthalmol Dec 2004; 52(4):271-80; PMID:15693317.
- Kennedy KD, AnithaChristy SA, Buie LK, Borras T. Cystatin a, a potential common link for mutant myocilin causative glaucoma. PloS One 2012; 7(5):e36301; PMID:22615763.
- Borras T. The effects of myocilin expression on functionally relevant trabecular meshwork genes: a mini-review. J Ocul Pharmacol Ther. Mar–Apr 2014; 30(2–3):202-12.
- Wentz-Hunter K, Shen X, Okazaki K, Tanihara H, Yue BY. Overexpression of myocilin in cultured human trabecular meshwork cells. Exp Cell Res Jul 1 2004; 297(1):39-48; PMID:15194423.
- Wang DY, Ray A, Rodgers K, Ergorul C, Hyman BT, Huang W, Grosskreutz CL. Global gene expression changes in rat retinal ganglion cells in experimental glaucoma. Invest Ophthalmol Vis Sci Aug 2010; 51(8):4084-95; PMID:20335623.
- Kirwan RP, Leonard MO, Murphy M, Clark AF, O'Brien CJ. Transforming growth factor-beta-regulated gene transcription and protein expression in human GFAP-negative lamina cribrosa cells. Glia Dec 2005; 52(4):309-24; PMID:16078232.
- Dong Z, Zhao X, Tai W, Lei W, Wang Y, Li Z, Zhang T. IL-27 attenuates the TGF-beta1-induced proliferation, differentiation and collagen synthesis in lung fibroblasts. Life Sci Feb 1 2016; 146:24-33; PMID:26776832; http://dx.doi.org/10.1016/j.lfs.2016.01.004
- Suzuki K, Satoh K, Ikeda S, Sunamura S, Otsuki T, Satoh T, Kikuchi N, Omura J, Kurosawa R, Nogi M, et al. Basigin promotes cardiac fibrosis and failure in response to chronic pressure overload in mice. Arterioscler Thromb Vasc Biol Feb 25 2016; 36:636-46.
- Tamm ER, Fuchshofer R. What increases outflow resistance in primary open-angle glaucoma? Surv Ophthalmol Nov 2007; 52 Suppl 2:S101-104; PMID:17998032.
- Hocevar BA, Howe PH. Analysis of TGF-beta-mediated synthesis of extracellular matrix components. Methods Mol Biol 2000; 142:55-65.
- Guo L, Moss SE, Alexander RA, Ali RR, Fitzke FW, Cordeiro MF. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest Ophthalmol Vis Sci Jan 2005; 46(1):175-82; http://dx.doi.org/10.1167/iovs.04-0832
- Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med Jan–Mar 2005; 9(1):59-71; http://dx.doi.org/10.1111/j.1582-4934.2005.tb00337.x
- Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. Nov 14 2005; 24(50):7455-64; PMID:16288292; http://dx.doi.org/10.1038/sj.onc.1209085
- Baleriola J, Garcia-Feijoo J, Martinez-de-la-Casa JM, Fernandez-Cruz A, de la Rosa EJ, Fernandez-Durango R. Apoptosis in the trabecular meshwork of glaucomatous patients. Mol Vis 2008; 14:1513-6; PMID:18728789.
- Levkovitch-Verbin H, Harizman N, Dardik R, Nisgav Y, Vander S, Melamed S. Regulation of cell death and survival pathways in experimental glaucoma. Exp Eye Res Aug 2007; 85(2):250-8; PMID:17586494; http://dx.doi.org/10.1016/j.exer.2007.04.011
- Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nature cell biology. Nov 2008; 10(11):1356-64; PMID:18931661; http://dx.doi.org/10.1038/ncb1795
- Agca C, Gubler A, Traber G, Beck C, Imsand C, Ail D, Caprara C, Grimm C. p38 MAPK signaling acts upstream of LIF-dependent neuroprotection during photoreceptor degeneration. Cell Death Dis 2013; 4:e785; PMID:24008729; http://dx.doi.org/10.1038/cddis.2013.323
- Zhang X, Schroeder A, Callahan EM, Coyle BM, Wang N, Erickson KA, Schuman JS, Fini ME. Constitutive signalling pathway activity in trabecular meshwork cells from glaucomatous eyes. Exp Eye Res Jun 2006; 82(6):968-73; PMID:16516195; http://dx.doi.org/10.1016/j.exer.2005.11.001
- Cho K-S, Chen DF. Optic nerve neuropathy and repair in glaucoma. N Am J Med Sci Oct 2008; 1(1):1; http://dx.doi.org/10.7156/v1i1p001
- Vrabec JP, Levin LA. The neurobiology of cell death in glaucoma. Eye Dec 2007; 21 Suppl 1:S11-14.
- Itakura T, Peters DM, Fini ME. Glaucomatous MYOC mutations activate the IL-1/NF-kappaB inflammatory stress response and the glaucoma marker SELE in trabecular meshwork cells. Mol Vis 2015; 21:1071-84; PMID:26396484.
- Wu Y, Chen W, Guo M, He Q, Hu Y. Effects of transforming growth factor-beta2 on myocilin expression and secretion in human primary cultured trabecular meshwork cells. Int J Clin Exp Pathol 2014; 7(8):4827-36; PMID:25197353.
- Xu B, Ju Y, Song G. Role of p38, ERK1/2, focal adhesion kinase, RhoA/ROCK and cytoskeleton in the adipogenesis of human mesenchymal stem cells. J Biosci Bioeng May 2014; 117(5):624-31; PMID:24331979; http://dx.doi.org/10.1016/j.jbiosc.2013.10.018
- Bradley JM, Kelley MJ, Rose A, Acott TS. Signaling pathways used in trabecular matrix metalloproteinase response to mechanical stretch. Invest Ophthalmol Vis Sci Dec 2003; 44(12):5174-181.; http://dx.doi.org/10.1167/iovs.03-0213
- Zhang Y, Ikegami T, Honda A, Miyazaki T, Bouscarel B, Rojkind M, Hyodo I, Matsuzaki Y. Involvement of integrin-linked kinase in carbon tetrachloride-induced hepatic fibrosis in rats. Hepatology Sep 2006; 44(3):612-22; PMID:16941698; http://dx.doi.org/10.1002/hep.21315
- Serrano I, McDonald PC, Lock FE, Dedhar S. Role of the integrin-linked kinase (ILK)/Rictor complex in TGFbeta-1-induced epithelial-mesenchymal transition (EMT). Oncogene Jan 3 2013; 32(1):50-60; PMID:22310280; http://dx.doi.org/10.1038/onc.2012.30
- Madala SK, Schmidt S, Davidson C, Ikegami M, Wert S, Hardie WD. MEK-ERK pathway modulation ameliorates pulmonary fibrosis associated with epidermal growth factor receptor activation. Am J Respirat Cell Mol Biol Mar 2012; 46(3):380-8; PMID:22021337; http://dx.doi.org/10.1165/rcmb.2011-0237OC
- Gupta VK, You Y, Li JC, Klistorner A, Graham SL. Protective effects of 7,8-dihydroxyflavone on retinal ganglion and RGC-5 cells against excitotoxic and oxidative stress. J Mol Neurosci Jan 2013; 49(1):96-104; PMID:23054592; http://dx.doi.org/10.1007/s12031-012-9899-x
- Sebe A, Masszi A, Zulys M, Yeung T, Speight P, Rotstein OD, Nakano H, Mucsi I, Szászi K, Kapus A. Rac, PAK and p38 regulate cell contact-dependent nuclear translocation of myocardin-related transcription factor. FEBS Lett Jan 23 2008; 582(2):291-8; PMID:18154735; http://dx.doi.org/10.1016/j.febslet.2007.12.021
- Takahashi E, Inoue T, Fujimoto T, Kojima S, Tanihara H. Epithelial mesenchymal transition-like phenomenon in trabecular meshwork cells. Exp Eye Res Jan 2014; 118:72-9; PMID:24291802; http://dx.doi.org/10.1016/j.exer.2013.11.014
- Swaney JS, Chapman C, Correa LD, Stebbins KJ, Bundey RA, Prodanovich PC, Fagan P, Baccei CS, Santini AM, Hutchinson JH, et al. A novel, orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br J Pharmacol Aug 2010; 160(7):1699-713; PMID:20649573; http://dx.doi.org/10.1111/j.1476-5381.2010.00828.x
- Pyne NJ, Dubois G, Pyne S. Role of sphingosine 1-phosphate and lysophosphatidic acid in fibrosis. Biochimica et biophysica acta. Jan 2013; 1831(1):228-38; PMID:22801038; http://dx.doi.org/10.1016/j.bbalip.2012.07.003
- Gonzalez-Ramos M, de Frutos S, Griera M, Luengo A, Olmos G, Rodriguez-Puyol D, Calleros L, Rodriguez-Puyol M. Integrin-linked kinase mediates the hydrogen peroxide-dependent transforming growth factor-beta1 up-regulation. Free Radical Biol Med Aug 2013; 61:416-27; PMID:23624332; http://dx.doi.org/10.1016/j.freeradbiomed.2013.04.029
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature Oct 9 2003; 425(6958):577-84; PMID:14534577; http://dx.doi.org/10.1038/nature02006
- Inoue-Mochita M, Inoue T, Fujimoto T, Kameda T, Awai-Kasaoka N, Ohtsu N, Kimoto K, Tanihara H. p38 MAP kinase inhibitor suppresses transforming growth factor-beta2-induced type 1 collagen production in trabecular meshwork cells. PloS One 2015; 10(3):e0120774; PMID:25799097; http://dx.doi.org/10.1371/journal.pone.0120774
- McDonnell F, O'Brien C, Wallace D. The role of epigenetics in the fibrotic processes associated with glaucoma. J Ophthalmol 2014; 2014:750459; PMID:24800062; http://dx.doi.org/10.1155/2014/750459
- Kirwan RP, Wordinger RJ, Clark AF, O'Brien CJ. Differential global and extra-cellular matrix focused gene expression patterns between normal and glaucomatous human lamina cribrosa cells. Mol Vis 2009; 15:76-88; PMID:19145252.
- Pokrovskaya O, Wallace DM, O'Brien CJ. Modulation of the fibrotic response of the human lamina cribrosa cell to cyclical mechanical stretch using the Rho kinase inhibitor Y-27632. Invest Ophthalmol Vis Sci2014; 55(13):5032-2.
- Wordinger RJ, Sharma T, Clark AF. The role of TGF-beta2 and bone morphogenetic proteins in the trabecular meshwork and glaucoma. J Ocul Pharmacol Ther Mar–Apr 2014; 30(2–3):154-62; http://dx.doi.org/10.1089/jop.2013.0220
- Johnson EC, Doser TA, Cepurna WO, Dyck JA, Jia L, Guo Y, Lambert WS, Morrison JC. Cell proliferation and interleukin-6-type cytokine signaling are implicated by gene expression responses in early optic nerve head injury in rat glaucoma. Invest Ophthalmol Vis Sci Jan 2011; 52(1):504-18; PMID:20847120; http://dx.doi.org/10.1167/iovs.10-5317
- Villarreal G, Jr., Chatterjee A, Oh SS, Oh DJ, Kang MH, Rhee DJ. Canonical wnt signaling regulates extracellular matrix expression in the trabecular meshwork. Invest Ophthalmol Vis Sci Nov 2014; 55(11):7433-40; http://dx.doi.org/10.1167/iovs.13-12652
- Sethi A, Mao W, Wordinger RJ, Clark AF. Transforming growth factor-beta induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci Jul 2011; 52(8):5240-50; http://dx.doi.org/10.1167/iovs.11-7287
- Sander EA, Downs JC, Hart RT, Burgoyne CF, Nauman EA. A cellular solid model of the lamina cribrosa: mechanical dependence on morphology. J Biomecham Eng Dec 2006; 128(6):879-89; PMID:17154690.
- Rao US. Diagnosing, preventing, and treating glaucoma. Virtual Mentor 2010; 12(12):934-7; PMID:23186819; http://dx.doi.org/10.1001/virtualmentor.2010.12.12.cprl1-1012
- Berdahl JP, Allingham RR, Johnson DH. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology May 2008; 115(5):763-8; PMID:18452762; http://dx.doi.org/10.1016/j.ophtha.2008.01.013
- Edwards ME, Good TA. Use of a mathematical model to estimate stress and strain during elevated pressure induced lamina cribrosa deformation. Curr Eye Res 2001; 23(3):215-25; PMID:11803484; http://dx.doi.org/10.1076/ceyr.23.3.215.5460
- Alexandrova E, Nassa G, Corleone G, Buzdin A, Aliper AM, Terekhanova N, Shepelin D, Zhavoronkov A, Tamm M, Milanesi L. Large-scale profiling of signalling pathways reveals an asthma specific signature in bronchial smooth muscle cells. Oncotarget Feb 5 2016 [epub ahead of print]; PMID:26863634.
- Makarev E, Cantor C, Zhavoronkov A, Buzdin A, Aliper A, Csoka AB. Pathway activation profiling reveals new insights into age-related macular degeneration and provides avenues for therapeutic interventions. Aging Dec 2014; 6(12):1064-75; PMID:25543336; http://dx.doi.org/10.18632/aging.100711
- Ethier CR, Read AT, Chan D. Biomechanics of Schlemm's canal endothelial cells: influence on F-actin architecture. Biophys J Oct 2004; 87(4):2828-37; PMID:15454474; http://dx.doi.org/10.1529/biophysj.103.038133
- Minckler DS. Histology of optic nerve damage in ocular hypertension and early glaucoma. Surv Ophthalmol Apr 1989; 33 Suppl:401-2; discussion 409–411; PMID:2473536.
