ABSTRACT
Silent information regulator type-1 (SIRT1) is the best-studied member of the Sirtuin (Sir2) family of nicotinamide dinucleotide (NAD)-dependent class III histone deacetylases (HDACs). Rrecently, it is suggested that SIRT1 may be involved in the development of malignant tumors including mouse lymphoma, but has not yet been explored in Angioimmunoblastic T-cell lymphoma (AITL). Therefore, we investigated the prevalence and the prognostic impact of SIRT1 expression in AITL. Immunohistochemical expression of SIRT1, p53 were evaluated by using a 2 mm core from 45 AITL patients. Positive expression of SIRT1 was seen in 71.11% (32 of 45) of patients and p53 expression were seen in 53.33% (24 of 45). SIRT1 and p53 expression were significantly associated with shorter PFS by univariate analysis (P=0.009 and P < 0.001, respectively), multivariate analysis also shows that SIRT1 expression relate to worse prognosis. We also suggest inferior survival in AITL with the combined expression of SIRT1 and clinical characteristics of high IPI scores, high clinical stage, increased serum LDH, decreased HGB and increased γ-Globulin. In conclusion, our results indicate that SIRT1 is strongly expressed in AITL and it act as a clinically significant prognostic indicator for AITL patients, may also serve as a therapeutic target in AITL.
KEYWORDS:
Introduction
Angioimmunoblastic T-cell lymphoma (AITL) is one of the most common forms of peripheral T-cell lymphoma (PTCL) and accounts for approximately 15–20 % of PTCL and 1–2 % of non-Hodgkin's lymphoma.Citation1,2 AITL generally affects the elderly population, and the median age of patients is greater than 60 y with a slighter greater preponderance in males. AITL is manifested mostly after exposure to medications such as specially antibiotics, viral, bacterial, fungal infection, or an allergic reaction suggesting abnormalities in immune regulation.Citation1 The most common symptom of AITL is generalized lymphadenopathy associating with B symptoms (fever, weight loss, night sweats).Citation3 And the following sign is hepatosplenomegaly. Bone marrow involvement and rash are approximately seen in up to 70% and 50% of AITL patients respectively.Citation4 Polyclonal hypergammaglobulinemia is also present in 50% of AITL patients.Citation2 At present, various therapeutic method have been developed, including steroids, cytotoxic chemotherapy, and immunomodulators, although the clinical course of AITL is aggressive and the prognosis of it is still poor. The median survival time is fewer than 3 y with conventional chemotherapies.Citation5 Despite recent progress in both chemotherapy and supportive care for hematological malignancies, AITL, compared to other non-Hodgkin lymphomas, pursues an aggressive clinical course, and the optimal therapeutic regimen remains to be determined. Moreover, AITL does not present any pertinent prognostic factor, except the achievement of a CR to therapy.Citation4 Many studies have focused on the significance of the intrinsic characteristics of the tumor, however, the clinical relevance of most of these markers is inconsistent and only a few of them retain sufficient prognostic significance.Citation6
Sirtuin1 (SIRT1) is a mammalian homolog of the saccharomyces cerevisiae chromatin-silencing factor Sir2 that functions as a nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase and belongs to the class III histone deacetylases,Citation7,8 it has been highlighted as a key regulator of lifespan extension by caloric restriction.Citation9 SIRT1 has the ability to deacetylation key cell cycle molecules and apoptosis regulatory proteins, such as p53, NF-kB, FOXO family proteins, and Ku70,Citation10,11 hence, it has playing a important role in various physiological processes, such as metabolism, apoptosis and aging.Citation10,11,12 SIRT1 has been regarded as a tumor promoter because recent reports reveal that it's expression is increased in various human malignant tumors, such as glioblastoma, prostate cancer and primary colon cancer.Citation13,14,15 While SIRT1 contributes to preservation of genomic stability and takes part in prevention, retardation, and suppression of carcinogenesis.Citation16 Recent studies have indicated that the upregulation of SIRT1 in malignancies inactivates p53 by deacetylation, allowing cells proliferate with damaged of DNA such as mutations accumulate, this effect disrupts the control of cell cycle and then promotes tumor progression.Citation8,17
The prognostic factors of AITL remain deficient, and SIRT1 is suggested to be involved in the development of various malignant tumors including hematologic malignancy, and played an important role in tumorigenesis. Meanwhile, limited evidence is available concerning about the expression and distribution of SIRT1 in AITL at present, thus, we first assessed a series of 45 paraffin-embedded tissue sections by immunohistochemistry in order to investigate the prevalence SIRT1 expression in AITL and we also estimate the prognostic impact of SIRT1 in AITL patients.
Materials and methods
Patients and samples
The subjects in this study included 45 AITL patients who were enrolled in the Second Affiliated Hospital of Harbin Medical University P. R. of China from January 2011 to November 2015. All the patients was diagnosed according to the current World Health Organization criteria. Informed consent was provided according to the Declaration of Helsinki, and the study protocol was reviewed and approved by the Medical Ethical Committee of the 2nd affiliated hospital of Harbin medical university. Twenty-three of the patients received CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy, 20 of these patients completed 6 cycles of CHOP therapy, the other 3 patients changed their regimens to PIVE, DICE, COPE respectively after one course of CHOP. Seventeen patients were treated with 6 cycles of CHOPE chemotherapy, and 5 patients received 6 cycles of L-CHOP therapy. None of the patients received haematopoietic stem-cell transplantations. The patients were grouped according to their age, clinical stage, serum lactate dehydrogenase (LDH) levels, γ-Globulin levels, HGB levels, and international prognostic index (IPI) scores (low: score 0 to 2 vs. high: score 3 to 5).
Immunohistochemical staining
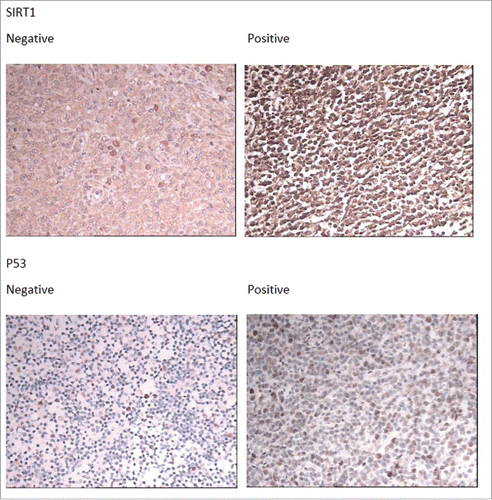
Immunohistochemistry was performed by using 2.0 mm cores for tissue microarray (TMA). The tissue sections were treated with a microwave antigen retrieval procedure in sodium citrate buffer for 12 minutes. The following markers were used: SIRT1 (1:50, Bioworld Technology, US), p53 (1:50, Novocastra, clone DO-7, Newcastle, UK). Images were acquired using a Nikon ECLIPSE E600 microscope (Nikon, Japan) with 40 objective lens (Plan Apo40/0.95 NA, Nikon, Japan) and digital camera (Nikon DXm1200F, Nikon, Japan) and software (Nikon ACT-1 2.62, Nikon, Japan). Immunostaining for SIRT1 and p53 were considered positive if 30% or more of the tumor cells were stained with an antibody.
Statistical analysis
Estimates of PFS distributions were calculated using the Kaplan-Meier method and comparisons of clinical and prognostic factors were performed using the log-rank test. Univariate and multivariate analyses were performed with a Cox hazards regression model using stepwise selection. The results are expressed as a hazard ratio (HR) and 95 % confidence interval (CI). All probability values were 2-sided and had an overall significance level of 0.05. All statistical analyses were performed using the version 20.0 SPSS software and graphpad prism 5 software.
Results
3.1 Clinical characteristics of patients
Forty-five patients were enrolled in the study. As shown in , the median age was 54.97 y (range 25–76 years). The female-to-male ratio was 1:2.2. Almost all patients (73.33%; 33 of 45) presented with advanced-stage disease and B symptoms were observed in 51.11%. At diagnosis, and splenomegaly and hepatomegaly were present in 42.22% (19 of 45) and 31.11% (14 of 45) of patients, respectively. Bone marrow infiltration were observed in 9(20%) patients. Laboratory investigations showed the presence of anemia in 28.89% (13 of 45), elevated serum LDH level in 57.78% (26 of 45), hypergammaglobulinemia occurred in 24.44% (11 of 45).
Table 1. Analysis of clinical characteristics as prognostic factors for PFS.
3.2 Immunophenotypical and biological characteristics
Immunohistochemical examinations of tumor cells showed that CD2 was positive in 28.89% (13 of 45), CD3 in 97.78% (44 of 45), CD4 in 64.44% (29 of 45), CD10 in 77.78% (35 of 45), CD20 in 95.56% (43 of 45), CD21 in 95.56% (43 of 45), CD30 in 82.22% (37 of 45), bcl-6 in 88.89% (40 of 45) and CXCL-13 in 93.33% (42 of 45) of the examined cases. And we used EBER-ISH to studied EBV, finding 34(75.56%) contained varying numbers of EBER positive cells.
3.3 SIRT1 and p53 protein expression in AITL
Expression of SIRT1 in AITL tissues by immunohistochemical staining are shown in . SIRT1 immunoreactivity was found primarily in the nuclei. Positive expression of SIRT1 was seen in 71.11% (32 of 45) of the patients and p53 in 53.33% (24 of 45). () And there is a significant correlation between SIRT1 expression and p53 expression (Spearman'r = 0.388, P = 0 .08).
3.4 Expression of SIRT1 in AITL correlated with reduced PFS univariate analysis
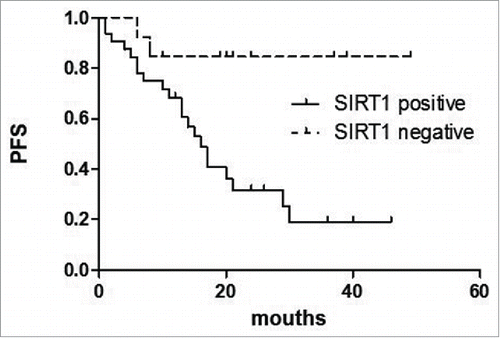
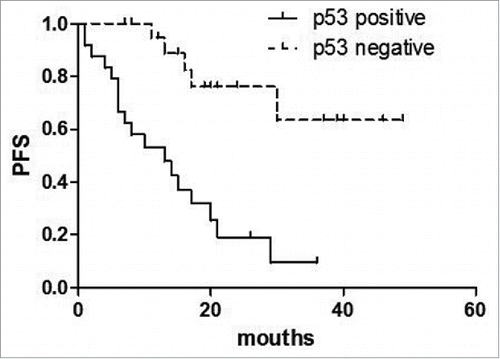
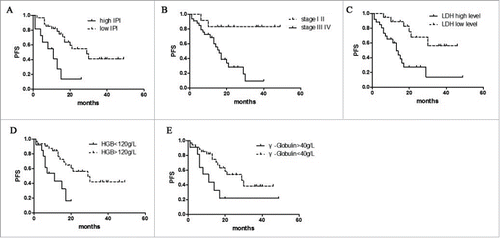
Univariate analysis of the expression of each protein and its relationship to PFS is shown in . For AITL in general, high IPI scores, high clinical stage, increased serum LDH, decreased HGB and increased γ-Globulin predicted shorter PFS(P = 0.005, p = 0.001, p = 0.002, p = 0.003, p = 0.032 respectively).() The expression of SIRT1 was associated with a significantly shorter PFS (P = 0.009) (). Similarly, p53 expression predicted shorter PFS (P < 0.001) ().
Figure 2. Survival analysis in AITL. The effect of LDH level, HGB level, γ-Globulin level, and international prognostic index (IPI) to PFS. High IPI scores (A), high clinical stage(B), increased serum LDH(C), decreased HGB(D) and increased γ-Globulin(E) predicted shorter PFS.
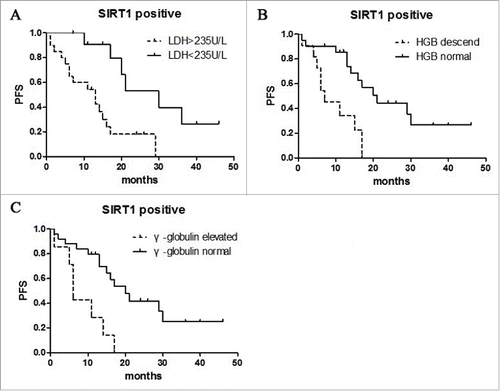
3.5 Survival analysis of SIRT1 combine with other clinical characteristics
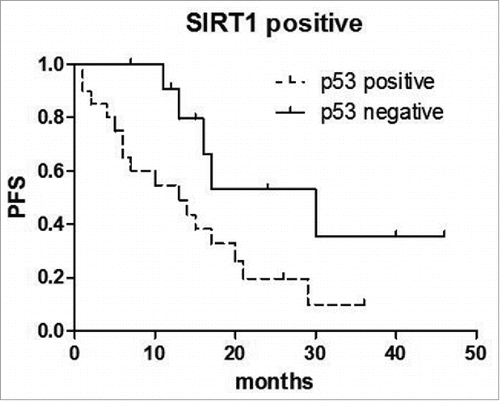
We find when confined to the cases of SIRT1 positive, the combined of p53 positive was significantly associated with a worse survival (p = 0.0326) (). And similarly, when confined to the SIRT1 positive cases, the combined of LDH (p = 0.0023), HGB (p = 0.0020), γ-Globulin (p = 0.0015) were significantly associated with worse survival (). These results suggest that inferior survival in AITL with the combined expression of SIRT1 and some clinical characteristics.
3.6 Multivariate analysis of SIRT1 expression in AITL
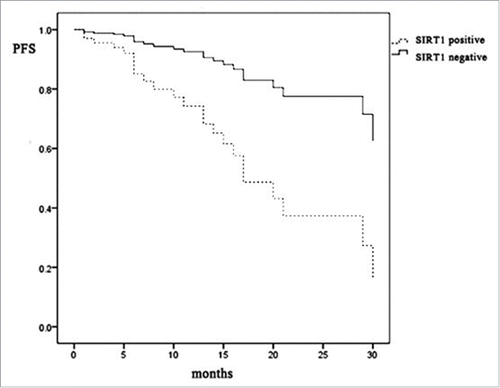
Multivariate analysis was performed using 45 patients with complete information for all variables. In , there is no significant item that can predict the prognosis by Multivariate Analysis, but as we can see in , the PFS of SIRT1 positive patient group is obvious lower than the SIRT1 negative group, suggesting SIRT1 expression relate to worse prognosis. Thus, SIRT1 expression can act as a prognostic indicator in AITL.
Table 2. Multivariate Cox Regression Analysis for PFS.
Discussion
This study demonstrated that (1) a high proportion of AITL (71.11%) demonstrated expression of SIRT1; (2) SIRT1 expression was associated with a significantly shorter PFS in AITL; (3) SIRT1 expression correlated with expression of p53 in AITL. These findings demonstrate that SIRT1 may be involved in the development of AITL and SIRT1 expression can act as a clinically significant prognostic indicator for AITL patients. Increased expression of SIRT1 has been reported in various malignant tumors,Citation13 however, the role of SIRT1 in AITL has not been evaluated previously. Therefore, this is the first report that explored the expression of SIRT1 in human AITL and its prognostic significance.
SIRT1 has been implicated in various pathophysiological processes including aging, apoptosis, senescence, metabolism, and neurodegenerative disorders.Citation12,18 SIRT1 has a variety of substrates such as NF-κB, Ku70, p53, E2F1, TFR2, PPARγ, p73, BcL-XL, MyoD, AR, cI AP2, and FOXO family of transcription factors.Citation19,20 Moreover, the function of many these substrate proteins is post-translationally regulated through an acetylation/deacetylation mechanism.Citation21 P53 as the most representative substrate of SIRT1 substrate, is considering an tumor suppressor, and acetylated-p53 is the active form of p53.Citation22 P53 can be activated by DNA damage triggers G1 arrest in the presence of growth factors and up-regulates a potential tumor suppressor microRNA 34a (miR-34a), inducing apoptosis. While, up-regulation of SIRT1 during the tumor development deacetylates and inactivates p53 of residue Lys382 in cancerous cells, leading to repression of miR-34a, DNA damage cells survive and bypass apoptosis, promoting tumor cell survival and tumorigenesis.Citation16,17,19,23 Furthermore, 2 functional p53 binding sites within the SIRT1 promoter (−178 bp and −168 bp) have been identified which normally repress SIRT1 expression. In the absence of nutrients, SIRT1 transcription is induced through nuclear translocation of FOXO3a, which interacts with p53 and thereby inhibits p53 suppressive activity.Citation24 Thus, we suggest that SIRT1 has a role in the development of AITL by inducing deacetylation of p53.
On the other hand, several studies suggest that SIRT1 serves as a tumor suppressor.Citation15 SIRT1 exerts anticarcinogenic effects through multiple mechanisms, for example, SIRT1 can counteract oxidative DNA damage , blocking initiation of carcinogenesis. And SIRT1 is also required for DNA repair processes to maintain genomic stability.Citation16,25 In general, SIRT1 takes part in prevention, retardation, and suppression of carcinogenesis. SIRT1 might act in both positive and negative effect to tumorigenesis, and it may depend on its subcellular localization.Citation16
Previous study has proved that SIRT1 expression is a clinically significant, poor prognostic indicator for patients with diffuse large B-cell lymphoma.Citation6 Other research find that SIRT1 is highly expressed in primary acute-type ATL cells, and may be a significant marker for the conversion to acute-type ATL.Citation26 In our study, the expression of SIRT1 is up-regulated in AITL patients, and associated with a significantly shorter PFS by univariate analysis, and multivariate analysis also shows that SIRT1 expression relate to worse prognosis.
AITL always pursues an aggressive clinical course, and it does not present any pertinent prognostic factor.Citation4 Many studies have focused on the significance of the intrinsic characteristics of the tumor, however, the clinical relevance of most of these markers is inconsistent and only a few of them retain sufficient prognostic significance. According to conventional cytogenetics of AITL, clonal aberrations are detected in up to 90%, and the most common recurrent abnormalities are trisomies of chromosomes 3, 5 and 21, gain of X and loss of 6q. But specific chromosomal abnormalities do not seem to be associated with survival, while complex karyotypes may adversely impact the outcome.Citation4 The International Prognostic Index (IPI) and the prognostic model for peripheral T-cell lymphoma (PIT) are useful clinical tools for aggressive lymphoma and PTCL, respectively. However, IPI was not predictive of survival in AITL patients, and a high PIT score was not associated with a good outcome.Citation2
Histone deacetylase (HDAC) inhibitors are a new class of antitumor agent that is currently undergoing intensive preclinical and clinical testing. Depsipeptides, a member of the cyclic peptide class of HDAC class I/II inhibitors, have shown cytotoxic effects on several malignant lymphoid cell lines. Significantly improved therapeutic efficacy of depsipeptide was reported in a clinical trial for the treatment of lymphoma in combination with daclizumab. Recent studies have demonstrated that HDAC class I/II inhibitors, MS-275, SAHA and LBH589, induce growth arrest and apoptosis of HTLV-1-infected T-cells via activation of p53.Citation26 Thus, HDAC inhibitors may be useful for treating patients with AITL or other types of cancer in which p53 plays a role, while the efficacy of HDAC class III inhibitors for AITL has not yet been reported.
In conclusion, a high percentage of AITL showed expression of SIRT1, and this expression of SIRT1 was correlated with the PFS of AITL patients. Because inhibitors of SIRT1 have anticancer potential, SIRT1, in addition to serving as a prognostic marker, may also serve as a therapeutic target in AITL.
Disclosure of potential conflicts of interest
The authors declare that they have no conflicts of interest to this work.
Funding
This work was supported by the Second Affiliated Hospital of Harbin Medical University.This work was supported by grants from the Postdoctoral Science Foundation of China (2015M580270), Postdoctoral Science Foundation of Heilongjiang Province (LBH-Z15129), National Natural Science Foundation of China (81001051), and New Century Excellent Talents in University (1252-NCET-014).
References
- Mosalpuria K, Bociek RG, Vose JM. Angioimmunoblastic T-cell lymphoma management. Semin Hematol 2014; 51(1):52-8; PMID:24468316; http://dx.doi.org/10.1053/j.seminhematol.2013.11.008
- Kameoka Y, Takahashi N, Itou S, Kume M, Noji H, Kato Y, Ichikawa Y, Sasaki O, Motegi M, Ishiguro A, et al. Analysis of clinical characteristics and prognostic factors for angioimmunoblastic T-cell lymphoma. Int J Hematol 2015; 101(6):536-42; PMID:25739382; http://dx.doi.org/10.1007/s12185-015-1763-7
- Frizzera G, Moran EM, Rappaport H. Angio-immunoblastic lymphadenopathy. Diagnosis and clinical course. Am J Med 1975; 59(6):803-18; PMID:1190254; http://dx.doi.org/10.1016/0002-9343(75)90466-0
- de Leval L, Gisselbrecht C, Gaulard P. Advances in the understanding and management of angioimmunoblastic T-cell lymphoma. Br J Haematol 2010; 148(5):673-89; PMID:19961485; http://dx.doi.org/10.1111/j.1365-2141.2009.08003.x
- Dogan A, Attygalle AD, Kyriakou C. Angioimmunoblastic T-cell lymphoma. Br J Haematol 2003; 121:681-91
- Jang KY, Hwang SH, Kwon KS, Kim KR, Choi HN, Lee NR, Kwak JY, Park BH, Park HS, Chung MJ, et al. SIRT1 expression is associated with poor prognosis of diffuse large B-cell lymphoma. Am J Surg Pathol 2008; 32:1523-31; PMID:18724249; http://dx.doi.org/10.1097/PAS.0b013e31816b6478
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem 2004; 73:417-35; PMID:15189148; http://dx.doi.org/10.1146/annurev.biochem.73.011303.073651
- Voelter-Mahlknecht S, Mahlknecht U. Cloning, chromosomal characterization and mapping of the NAD-dependent histone deacetylases gene sirtuin 1. Int J Mol Med 2006; 17:59-67; PMID:16328012
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol 2005; 6:298-305; PMID:15768047; http://dx.doi.org/10.1038/nrm1616
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 2001; 107:137-48; PMID:11672522; http://dx.doi.org/10.1016/S0092-8674(01)00524-4
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2 (SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001; 107:149-59; PMID:11672523; http://dx.doi.org/10.1016/S0092-8674(01)00527-X
- Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J 2007; 404:1-13; PMID:17447894; http://dx.doi.org/10.1042/BJ20070140
- Chu F, Chou PM, Zheng X, Mirkin BL, Rebbaa A. Control of multidrug resistance gene mdr1 and cancer resistance to chemotherapy by the longevity gene sirt1. Cancer Res 2005; 65:10183-7; PMID:16288004; http://dx.doi.org/10.1158/0008-5472.CAN-05-2002
- Kuzmichev A, Margueron R, Vaquero A, Preissner TS, Scher M, Kirmizis A, Ouyang X, Brockdorff N, Abate-Shen C, Farnham P, et al. Composition and histone substrates of polycomb repressive group complexes change during cellular differentiation. Proc Natl Acad Sci USA 2005; 102:1859-64; PMID:15684044; http://dx.doi.org/10.1073/pnas.0409875102
- Deng CX. SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci 2009; 5:147-52; PMID:19173036
- Song NY, Surh YJ. Janus-faced role of SIRT1 in tumorigenesis. Ann N Y Acad Sci 2012; 1271:10-19; PMID:23050959; http://dx.doi.org/10.1111/j.1749-6632.2012.06762.x
- Lim CS. Human SIRT1: a potential biomarker for tumorigenesis? Cell Biol Int 2007; 31:636-7; PMID:17433727; http://dx.doi.org/10.1016/j.cellbi.2006.11.003
- Dai Y, Faller DV. Transcription Regulation by Class III Histone Deacetylases (HDACs)-Sirtuins. Transl Oncogenomics 2008; 3:53-65; PMID:21566744
- Moore RL, Dai Y, Faller DV. Sirtuin 1 (SIRT1) and steroid hormone receptor activity in cancer. J Endocrinol 2012; 213:37-48; PMID:22159506; http://dx.doi.org/10.1530/JOE-11-0217
- Nihal M, Ahmad N, Wood GS. SIRT1 is upregulated in cutaneous T-cell lymphoma, and its inhibition induces growth arrest and apoptosis. Cell Cycle 2014; 13(4):632-40; PMID:24343700; http://dx.doi.org/10.4161/cc.27523
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004; 303:2011-5; PMID:14976264; http://dx.doi.org/10.1126/science.1094637
- Frazzi R, Valli R, Tamagnini I, Casali B, Latruffe N, Merli F. Resveratrol-mediated apoptosis of hodgkin lymphoma cells involves SIRT1 inhibition and FOXO3a hyperacetylation. Int J Cancer 2013; 132(5):1013-21; PMID:22833338; http://dx.doi.org/10.1002/ijc.27748
- Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell 2005; 123:437-48; PMID:16269335; http://dx.doi.org/10.1016/j.cell.2005.08.011
- Zschoernig B, Mahlknecht U. SIRTUIN 1: Regulating the regulator. Biochem Biophys Res Commun 2008; 376:251-5; PMID:18774777; http://dx.doi.org/10.1016/j.bbrc.2008.08.137
- Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, Hartlerode A, Stegmuller J, Hafner A, Loerch P, et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 2008; 135:907-18; PMID:19041753; http://dx.doi.org/10.1016/j.cell.2008.10.025
- Kozako T, Aikawa A, Shoji T, Fujimoto T, Yoshimitsu M, Shirasawa S, Tanaka H, Honda S, Shimeno H, Arima N, et al. High expression of the longevity gene product SIRT1 and apoptosis induction by sirtinol in adult T-cell leukemia cells. Int J Cancer 2012; 131(9):2044-55; PMID:22322739; http://dx.doi.org/10.1002/ijc.27481