ABSTRACT
S100A4, a small intra- and extracellular Ca2+-binding protein, is involved in tumor progression and metastasis with S100A4 level shown to be correlated with tumor cells metastatic potential. Simultaneously, Octamer transcription factor 1 (Oct-1) regulates a wide range of genes and participates in tumor cell progression with high Oct-1 level associated with a poor prognosis for different tumors. In this study, following the establishment of Oct-1 binding site, we used Burkit lymphoma B cells (Namalwa cells) which express different isoforms of Oct-1 (Oct-1A, Oct-1L and Oct-1X) to investigate the role of Oct-1 in S100A4 expression and sustaining intra- and extra-cellular S100A4 levels. As antitumor agents, we used dexamethasone which effect is mediated by the activation of intracellular glucocorticoid receptors and camptothecin which molecular target is nuclear DNA topoisomerase I (TOP1). We established that, firstly, the most significant increase in S100A4 gene expression has been demonstrated in the cells transfected with Oct-1A. Secondly, we have established that high level of Oct-1 and decreased intracellular S100A4 level decline the survival of Namalwa cells under dexamethasone treatment. Thirdly, we have shown that the tumor cells transformation by different Oct-1 isoforms retained those cells' sensitivity to the antitumor effect of combined dexamethasone and camptothecin. In contrast, in the non-transformed Namalwa cells, dexamethasone decreased the camptothecin effect on the cells survivorship, thus, emphasizing Oct-1 role in the regulation of cell response to different antitumor agents. The results identify a necessity to consider Oct-1 level for combined chemotherapeutic drug treatment.
Introduction
One of the indicators of the most malignant tumors is their high metastatic activity. Metastatic potential of tumor cells manifests in several biomarkers, for instance via the expression of S100A4/Mts (11 kDa) protein belonging to S-100 protein family.Citation1
The molecular mechanisms of S100A4 involvement into tumor progression are defined by the protein localization. S100A4 protein contains both inside cells, mainly within cytoplasm, and is secreted into extracellular spaces. The role of intracellular S100A4 in tumor progression is associated with the interaction of that protein and cytoskeleton proteins, particularly with nonmuscle myosin heavy chain (NMMHC) IIA which leads to increased cell motility and invasiveness.Citation2 Specifically, the data demonstrates S100A4 participation in the induction of epithelial to mesenchymal transition (EMT) and, thus, the promotion of tumor cells invasiveness and motility.Citation3 Additionally, the intracellular S100A4 expression is associated with MMPs and E-cadherin genes regulation; however, the molecular mechanisms of that regulation are currently unknown.
The role of extracellular S100A4 in tumor progression is no less important. Extracellular S100A4 is secreted by both tumor and stromal cells. By interacting with annexin II (AII) and tissue plasminogen activator (tPA) on endothelial cells surface, S100A4 stimulates the conversion of plasminogen into plasmin and, hence, induces angiogenesis.Citation4 Additionally, by biding with RAGE receptor located on the cellular surface, S100A4 activates intracellular signal transduction cascades including mitogen-activated protein kinases which results in increased Ca2+ concentration within tumor cells cytoplasm. Consequently, cell motility, invasiveness, and angiogenesis altogether contribute to the stimulation of metastasis.Citation5
Unfortunately, the mechanism of S100A4 secretion as well as proteins controlling that process is currently unknown. The identification of that mechanism promises new opportunities for controlling tumor cells metastasis. Thus, in this study we investigated proteins stimulating S1004A secretion in tumor cells in order to strengthen our understanding of S100A4 turnover.
Likewise, the mechanisms regulating S100A4 transcription in cells are still being investigated. However, we have identified the site for Oct-1 transcription factor in s100a4 gene's regulatory region (ONCOMINE database) and, thus, decided to investigate the role of that factor in S100A4 transcription regulation.
Oct-1 (gene symbol POU2F1) is a member of DNA-binding POU domain containing group of proteins, which includes transcription regulators among higher eukaryotes.Citation6,7,8 Oct-1 controls the vast number of targets and is considered to be one of the important regulators of normal and tumor cell functioning. The high level of Oct-1 in tumor cells is strongly associated with poor survival of patients suffering from several malignant tumors.Citation9 The present data demonstrates that Oct-1 is a positive regulator of tumor progression by means of activating cell proliferation and repressing the genes related both to antigen processing and presentation and cytokine-cytokine receptor interaction. Oct-1 has multiple isoforms: the most studied are abundantly expressed Oct-1A and tissue-specific isoforms Oct-1L and Oct-1X.Citation10 The three isoforms differ by their N-terminal sequences and control the expression of different but overlapping sets of genes. Therefore, in the current study we investigated the role of different Oct-1 isoforms in S100A4 expression and secretion by tumor cells.
Finally, in our previous studies we demonstrated that the high level of S100A4 within tumor cells decreases their death rate caused by dexamethasone, a synthetic analog of glucocorticoid hydrocortisone.Citation11 Dexamethasone as a medication is included into standard treatment schemes of antitumor therapy with demonstrated inhibitory effects on lymphocytes proliferation during lymphoma and leukemia treatments. Additionally, in our previous studies we established that highly metastatic KSML-100 adenocarcinoma cells with increased S100A4 level were insensitive to dexamethasone effect. Moreover, Oct-1 was shown to participate in the maintenance of target cell specificity of glucocorticoid responsiveness.Citation12 Considering that glucocorticoids are also widely used as a co-medication in cancer therapy for malignant tumors, for instance in a combination with a chemotherapy agent, camptothecin,Citation13 we tested if tumor cells' sensitivity to dexamethasone retains under the dexamethasone-camptothecin combination. Taking in account the important role of glucocorticoids in suppressive therapy of lymphoid malignancies, in the current study, we additionally investigated the effects of different Oct-1 isoforms on the survival of malignant lymphoid Namalwa cells incubated with dexamethasone and dexamethasone-camptothecin.
Results
High level of oct-1 isoforms in the namalwa cells activates S100A4 transcription
The first goal of our study was to verify if Oct-1 isoforms influence S100A4 transcription. For our work Namalwa cell culture (Burkit lymphoma B cells) has been chosen since the expression of both S100A4 and Oct-1 was demonstrated in these cells. The computer analysis of DNA sequence for Mts1/S100A4 gene (http://www.genecards.org) identified an Oct-1 binding site 3.5 kbp upstream of start transcription. That finding allowed considering an Oct-1 regulatory function in S100A4 expression. We suggested that Oct-1 may participate in regulation of S100A4 transcription level. Consequently, the presence of Oct-1 on its binding site upstream of S100A4 in the Namalwa cells was verified by chromatin immunoprecipitation (). The level of Oct-1 on intron region of CD3 gene was used as a control. The ChIP experiments demonstrated the presence of significant amount of Oct-1 on the corresponding site in the Namalwa cells. All overexpressed isoforms were also found at this site. The highest affinity was observed for Oct-1A isoform. Thus, the endogenous form of Oct-1 as well as the overexpressed isoforms are present at the Oct-1 binding site upstream of S100A4 and may participate in its regulation.
Figure 1. ChIP analysis of Oct-1 at the 5′-CGCCCTGCGTATTCCT-3′ (S100A4) site of the regulatory region of s100a4 gene. The data was quantified and normalized to the level of gene CD3 used as a control. Columns: (1) Namalwa – non-transformed Namalwa cells, (2) NOct1A, (3) NOct1L, (4) NOct1X – the Namalva cells transformed with Oct-1A, Oct-1L, and Oct-1X isoforms respectively. Namalwa – ChIP was performed with anti- Oct-1 antibodies; NOct1A, NOct1L, NOct1X – ChIP was performed with anti-FLAG antibodies. The data corresponds to single biological samples analyzed in triplicates. B. The effect of Oct-1 overexpression on S100A4 transcription level. The relative amounts of mRNA in the transformed cells were quantified and normalized to the corresponding mRNA amounts in the non-transformed cells. The relative transcription level of S100A4 mRNAs in the cells was measured by Real-Time PCR. Error bars show SEM based on 5 biological replicates. A, B *p < 0.05.
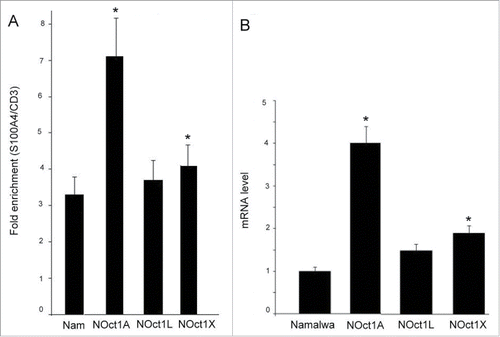
Further, we investigated the effect of Oct-1 overexpression on S100A4 transcription level (). Our data demonstrated that S100A4 transcription level correlates with the level of Oct-1 isoform on its binding site (r = 0.77; p < 0.05). Specifically, the S100A4 level increases in 1.5 and 2 times respectively for Oct-1L and Oct-1X isoforms but rises 4-fold in the cells transfected with Oct-1A. All Oct-1 isoforms activate S100A4 gene with the greatest effect observed for Oct-1A, the isoform demonstrated to be expressed in all human tissues.Citation14 Therefore, we have established a positive correlation between S100A4 mRNA level () and Oct-1 enrichment () regardless of Oct-1 isoform.
High level of Oct-1 isoforms in the namalwa cells influences S100A4 secretion
In order to identify a correlation between Oct-1 transcription level and S100A4 expression, we measured S100A4 level in Namalwa cells transformed with different Oct-1 isoforms. Our data demonstrated that intracellular S100A4 level is significantly decreased in the cells transformed with Oct-1 isoforms in comparison with the non-transformed Namalwa cells (). Since these changes did not correlate with the increase of Oct-1 transcription level, we suggested that high level of Oct-1 may also stimulate S100A4 secretion. Indeed, we observed the significant increase of S100A4 in the condition medium of cells transformed with Oct-1 isoforms. Thus, we concluded that the increase of total amount of S100A4 in transformed cells correlates with the increase of Oct-1 transcription. However, S100A4 intercellular level goes down due to the increase of S100A4 secretion in Oct-1 transformed cells.
Figure 2. The effect of Oct-1 overexpression on S100A4 protein level in the Namalwa cells (intracellular) and protein secreted by the cells into the culture medium (extracellular). The S100A4 content in the samples was measured by ELISA. NOct1A, NOct1L, NOct1X – Namalwa cells transformed with Oct-1A, Oct-1L, Oct-1X isoforms. Error bars show SEM based on 5 biological replicates. *p < 0.05 B. The effect of Oct-1 overexpression on intracellular S100A4 protein level. Total protein was extracted from the intact Namalwa cells and Namalwa cells transformed with Oct-1A, Oct-1L, Oct-1X isoforms (NOct1A, NOct1L, NOct1X ) and western blotting was performed using antibodies against S100A4. C. Western blot analysis of materials from the Namalwa cells. Intracellular S100A4 protein (cells) and S100A4 secreted into the condition medium (c.m.).
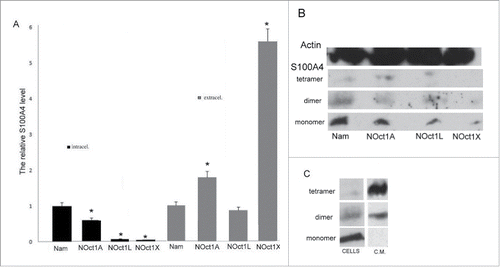
The analysis of the correlation between intracellular levels of S100A4 and S100A4 secretion in the cells transformed by different Oct-1 isoforms demonstrated that S100A4 secretion is not directly dependent on S100A4 concentration in the cells. For instance, the 4-fold increase in S100A4 transcription for Oct-1A Namalwa cells leads to the increase of S100A4 level in the medium in less than to 2 times. At the same time, the 2-fold increase in S100A4 transcription for Oct-1X isoform leads to S100A4 level increased in almost 6 times. No increase of S100A4 secretion was observed under over-expression of Oct-1L.
In addition, Oct-1 isoforms have different influence on S100A4 transcription and secretion. While Oct-1A induces the S100A4 transcription the most, Oct-1X is the most efficient in the stimulation of S100A4 secretion into the condition medium. We propose that specific Oct1 isoforms participate in the regulation of S100A4 transcription and secretion to different extents and that these 2 processes induced by Oct-1 are likely independent of each other.
S100A4 protein is known to exist in both monomeric and oligomeric forms with the oligomeric form being a functional form secreted extracellularly. Western blot analysis revealed that the intracellular protein in Namalwa cells is mostly present in monomeric form (10 kDa) and to a lesser extent in dimeric (20 kDa) and tetrameric (40 kDa) forms. At the same time, in the condition medium the protein is present as a dimer or as a tetramer ().
Further we investigated how the increased Oct-1 level effects the distribution of S100A4 protein forms within the cells.
Co-facilitator role of dexamethasone and Oct-1L/Oct-1X in extracellular and intracellular distribution of S100A4 protein
Our previous studies demonstrated that high level of S100A4 within tumor cells decreases their death rate caused by dexamethasone.Citation11 Dexamethasone, most commonly used to prevent or decrease the side effects of chemotherapy and radiation, has also shown an anti-cancer activity when used alone (for example, in cases of acute lymphoblastic leukemia, lymphoma, and multiple myeloma) or in combination with chemotherapeutic agents. We used dexamethasone, a synthetic glucocorticoid agonist, which demonstrates the characteristics different from those of other glucocorticoid agonists (hydrocortisone and prednisolone). Specifically, dexamethasone binds to culture medium serum proteins in much lower levels than other glucocorticoids while demonstrating high glucocorticoid receptor affinity.Citation15 In addition, Oct-1 was shown to participate in the maintenance of target cell specificity of glucocorticoid responsiveness.Citation12 Thus, we investigated the influence of dexamethasone on S100A4 expression and secretion in cells with high level of Oct-1 expression. First, we established that Namalwa cells cultivation with dexamethasone did not modify those genes transcription levels (). At the same time, in Namalwa cell lines with overexpressed Oct-1 isoforms, dexamethasone enhanced the level of S100A4 transcription suggesting that at high concentration Oct-1 co-operates with dexamethasone in S100A4 activation (). The highest increase of the transcription was observed for the cells transformed with Oct-1L and Oct-1X isoforms: in 3 and 2.5 times respectively in comparison with control measurements.
Figure 3. The effect of dexamethasone on Oct-1 and S100A4 transcription levels in the Namalva cells. The relative transcription level of total Oct-1 and S100A4 mRNAs in the cells was measured by Real-Time PCR. B. The resulting effect of dexamethasone and Oct-1-overexpressing on S100A4 transcription level in the Namalva cells. The relative transcription level of total S100A4 mRNAs in the the dexamethasone-treated and in the non-dexamethasone-treated cells was measured by Real-Time PCR. A, B. Error bars show SEM from 5 separate experiments. C. The effect of dexamethasone and Oct-1-overexpressing on S100A4 intracellular level in the Namalva cells (intracellular) and on the secretion of S100A4 by Namalva cells (extracellular). The relative amounts of S100A4 protein in the dexamethasone-treated cells were quantified and normalized to the corresponding amounts of S100A4 protein in non-treated cells. The S100A4 content in the samples was measured by ELISA. The histograms are representative of 5 separate experiments. D. The effect of Oct-1 overexpression on cell viability; the cells treated with dexamethasone or camptothecin or both. Namalwa cell viability was scored by MTT assays. Relative survival was calculated by setting the values in the corresponding non-dexamethasone and non-camptothecin cells as 100%. The histograms are representative of 5 separate experiments. B, C, D *p < 0.05.
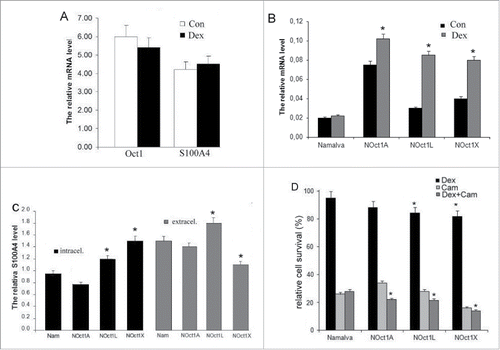
Further, we studied how high level of Oct-1 isoforms influences S100A4 extra- and intracellular level in the cells incubated with dexamethasone. In the intact Namalwa cells, intracellular S100A4 level did not change under the dexamethasone treatment (). In the cells expressing Oct-1 isoforms, we did not observe the influence of dexamethasone on intracellular content of S100A4 (). The maximum increase in S100A4 level under the dexamethasone treatment was observed for Oct-1X transformed cells – 1.5 times. Thus, intracellular S100A4 level decreased due to overexpression of Oct-1 isoforms did not change significantly under the dexamethasone effect.
Extracellular S100A4 secretion by the intact Namalwa cells cultivated with dexamethasone was increased in 1.5 times (). In turn, in the cells transformed with Oct-1 isoforms under the dexamethasone treatment, we observed the increase of S100A4 secretion comparable with the level in the intact cells. Specifically, for the cells transformed with Oct-1A – in 1.4 times and for the cells transformed with Oct-1L – in 1.8 times. Thus, the overexpression of Oct-1 isoforms did not influence the dexamethasone effect on S100A4 secretion with the exception of the cells transformed with Oct-1X where no reliable changes in the secretion were observed. Comparing with the intact Namalwa cells, these results indicate that Oct-1X overexpression someway represses the effect of dexamethasone on S100A4 secretion.
Summarizing these findings, we observed the co-facilitator of dexamethasone with both Oct-1L and Oct-1X in S100A4 protein expression level. Dexamethasone itself stimulates secretion of S100A4. The overexpression of Oct-1A and Oct-1L isoforms does not alter the secretion level of S100A4 stimulated by dexamethasone while Oct-1X has some repressive effect.
Oct-1 overexpression influence on Namalwa cells sensitivity to dexamethasone and combined effect of dexamethasone and camptothecin
Since dexamethasone demonstrates inhibitory effects on lymphocytes proliferation during lymphoma and leukemia treatments, it is included as a medication into standard treatment schemes of antitumor therapy. We investigated the effect of higher level of Oct-1 and increased S100A4 extracellular secretion on Namalwa cells' viability in the presence of dexamethasone. We observed that the number of living cells after 36-hour incubation with dexamethasone was lower for the cell cultures transformed with Oct-1 rather than for the intact Namalwa line (). The number of surviving cells transformed with Oct-1L and Oct-1X was reliable lower than in the intact cells and Namalwa cells stably expressing Oct-1A. The survival correlated with S100A4 intracellular level: for cells transformed with Oct-1A the level decreased in 1.7 times, for Oct-1L and Oct-1X plummeted in 13 and 20 times respectively ().
Nuclear DNA topoisomerase I (TOP1) is an essential human enzyme. TOP1 is targeted by camptothecins which include alkaloid camptothecin and its derivatives, anticancer agents, irinotecan and topotecan; however, camptothecin penetrates vertebrate cells readily and target TOP1 within minutes of exposure.Citation16 Camptothecins' adverse effects preclude their wide use in the medical practice. At the same time, glucocorticoids, such as dexamethasone, are widely used as a co-treatment with chemotherapy because of their effectiveness to prevent those adverse effects (edema, nausea, and toxic reaction) caused by cytotoxic treatment in tumor patients.Citation17 Thus, we tested if tumor cells' sensitivity to dexamethasone retains under the dexamethasone-camptothecin combination (). As expected, the number of surviving cells under camptothecin effect dramatically decreased in the intact Namalwa cells. The number of surviving cells under the camptothecin effect was the lowest in the cell line transformed by Oct-1X, the highest in the cell line transformed by Oct-1A, and remained at the level of the intact cells for the cell line transformed by Oct-1L.
The combined effect of dexamethasone and camptothecin on the intact Namalwa cells led to a modest increase in the number of surviving cells in comparison with camptothecin-only effect which concurs with the study demonstrating a decreased camptothecin effect if dexamethasone is also present.Citation13 In contrast, in all cell lines with over-expressed Oct-1, dexamethasone has increased camptothecin antitumor effect. Thus, the observed efficacy of glucocorticoids on cell viability correlates with decreased intracellular S100A4 level caused by overexpression of Oct-1 isoforms. Those findings agree with the previously established insensitivity to dexamethasone effect for cells with increased S100A4 level.Citation11
Discussion
In our study we demonstrated that the high expression levels of Octamer transcription factor (Oct-1) isoforms: Oct-1A, Oct-1L and Oct-1X - in tumor cells activate S100A4 gene transcription and lead to active secretion of S100A4 protein, a main biomarker of highly metastatic cells. At the same time, the decreased intracellular S100A4 level results in higher cells' sensitivity to dexamethasone lethal effect.
We suggest that the active S100A4 transcription is caused by Oct-1 biding with the regulatory region of S100A4 gene thus inducing the transcription process. It was demonstrated that Oct-1 regulates the transcription of target genes by interacting with both promoters and enhancers. For instance, Oct-1 activates the transcription of immunoglobulin genes by interacting with a heavy-chain enhancer.Citation18
Additionally, we demonstrated that simultaneous Oct-1 overexpression and cells cultivation with dexamethasone results in enhanced S100A4 gene activation. This may be explained by the interaction of Oct-1 and glucocorticoid receptor since the glucocorticoid receptor cultivated with its agonist, dexamethasone, is able to interact with DNA-bound Oct-1 which results in the regulation of genes transcription.Citation19,20 Like all glucocorticoids, dexamethasone interacts with a glucocorticoid receptor which is a member of the nuclear receptor superfamily. Multiple signaling pathways are involved following binding of the steroid to the receptor, including direct DNA binding which results in changes to gene transcription.Citation21 Thus, we propose that, in case of Oct-1 overexpression, the surplus of the recombinant protein also binds with the glucocorticoid receptor enhancing S100A4 gene activation.
We suggest that active S100A4 secretion which decreases intracellular S100A4 level causes higher dexamethasone lethality in cells due to the effect on chaperon proteins. The system of chaperon proteins Hsp90/Hsp70 was shown to determine the cell-targets sensitivity to glucocorticoids regulating functional activity of the glucocorticoid receptor.Citation22 In our previous studies we established that S100A4 binds with chaperone Hsp70 forming a non-active complex.Citation23 We propose that S100A4 disrupts the stability and functionality of GCGR complex by interrupting Hsp70 and Hsp90 interaction which results in decreasing apoptotic cell response to glucocorticoids. The decreased intracellular S100A4 level in cells with overexpressed isoforms Oct-1L and Oct-1X in our experiments is proposed to mitigate that disruptive effect of S100A4 on the dexamethasone-induced tumor cells death. Therefore, the increased levels of Oct-1L and particularly Oct-1X in maligned cells indicates the possibility of effective antitumor glucocorticoid therapy.
As for the increased level of extracellular S100A4, it was shown to lead to tumor growth progression.Citation24 At the same time, our earlier studies demonstrated that the increased level of extracellular S100A4 also leads to inhibited lymphocytes cytotoxic activity.Citation25 In our experiments the extracellular S100A4 level was significantly increased for Oct-1A and particularly Oct-1X isoforms. Thus, the active S100A4 secretion induced by overexpressed Oct-1X leads to increased lymphoid tumor malignization.
Summarily, the increased Oct-1 isoforms overexpression exerts oppositely directed effect on S100A4-mediated tumor cells progression. On one side, the overexpression increases S100A4 synthesis and extracellular concentration leading to tumor progression. On the other side, the overexpression decreases the S100A4 intracellular level leading to higher tumor cells sensitivity to glucocorticoids.
A synergetic effect of the dexamethasone-camptothecin combination leading to higher cells sensitivity to those agents is most probably based on the modification of S100A4 exchange between intra- and extracellular compartments. Since in the intact Namalwa cells dexamethasone was decreasing camptothecin effect on the cell's viability, Oct-1 plays an important role in the regulation of cell response to antitumor agents with different mechanisms of actions.
Our findings are especially important as during recent years the role of Oct-1 in tumor development has become more evident. Our study demonstrates an additional way Oct-1 influences tumor progression via stimulating the transcription level of S100A4 and its secretion by tumor cells. Moreover, as Oct-1-induced S100A4 overexpression and extracellular secretion is proposed to be an important mechanism regulating tumor cells sensitivity to glucocorticoids, Oct-1 isoforms could also be potential targets for the pharmacotherapeutic malignization control.
Materials and methods
Cell cultures and transduction of namalwa cells
Namalwa, human lymphoblastoid cells were grown in the suspension culture in RPMI 1640 medium supplemented with 10% fetal calf serum and antibiotics in a humidified incubator with 5% CO2 atmosphere at 37°C. Cell lines were maintained in DMEM with 10% FCS, penicillin 100 U/ml, and streptomycin 100 μg/ml. ViralPower Lentiviral Expression System (Invitrogen) was used for the stable transduction of Namalwa cells in accordance with the manufacturer's protocol. Blasticidin was used to maintain the stably transformed cells and was withdrawn from the medium 3 d before the experiment.
Constructs
The constructs pL-Oct-1A-3FLAG, pL-Oct-1L-3FLAG, and pL-Oct-1X-3FLAG were generated by inserting a copy of human Oct-1 coding sequences into pLenti6/V5-D-TOPO expression vector (Invitrogen). The Oct-1 transcript containing the open reading frame for Oct-1A protein which represents the longest form of Oct-1 including all internal exones (766 amino acids) was cloned in Human NM_002697. We have provided a GenBank accession number for the nucleotide sequence: SEQ1.sqn Portseva_2 SEQ1 KT438684. We have also demonstrated that Oct-1A, Oct-1L and Oct-1X are transcribed from alternative promoters of POU2F1 gene and the predicted isoforms differing by their N-terminus.Citation10,26,27
RNA purification and RT-PCR analysis
The expression level of Oct-1 and S100A4 was measured by RT-PCR. Total RNA was extracted from the cells using Rneasy Plus Mini Kit (Qiagen). The cDNA was synthesized with oligo-dT primer and 2 mkg RNA in 20 mkl volume. The following primers were used: Oct-1-total Forw 5′-AGGAGCAATCTCAACAGCCC-3′; Rev 5′- GACTGAGCAGCAGCCTGTAA-3′. The following primers were used for S100A4: Forw 5′-GCCCTGGATGTGATGGTGTCCA and Rev 5′-TCATGGCGATGCAGGACAGGA. Measurements at each point were made in at least 3 replications, and the mean value was calculated.
Computational analysis of gene regulatory regions
Potential transcription factor binding sites were predicted with NCBI (http://www/ncbi.nlm.nih.gov). Identification and analysis of the regulatory regions of S100A4 gene in Namalva cells was performed using the Primer3 (http://frodo.wi.mit.edu) and program Vector NTI (InforMax); GeneCard (http://www/genecard.org).
Determination of S100A4 in samples
The protein quantitation was made using the Quant-iT Protein Assay Kit (Invitrogen) and Qubit Fluorometer. S100A4 content in the samples was measured by competitive EIA.Citation28 Recombinant S100A4 in a concentration of 200 ng/ml was adsorbed in the wells of a 96-well plate. The reaction was carried out by adding 50 μl polyclonal rabbit antibodies to S100A4 (1:1000, Neo Markers) to 50 μl analyzed fluid. Recombinant S100A4 in concentrations of 1-1000 ng/ml was used for plotting the calibration curve.
Antibodies and immunoblotting
We used Rabbit polyclonal anti-S100A4 antibodies (Ab-8), NeoMarkers (Fremont, CA). Samples were resolved by 10–15% SDS-PAGE and blotted onto a PVDF membrane (GE Healthcare Ltd., UK). The membrane was blocked with 1% non-fat dry milk and incubated with primary antibodies and horseradish peroxidase-conjugated secondary antibodies (GE Healthcare). Proteins were visualized using ECL Plus detection reagents (GE Healthcare) in accordance with the manufacturer's protocol.
Dexamethasone and camptothecin treatment and MTT assay
We used CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega Corporation) as a colorimetric method for determining the number of viable cells. Cells were seeded in 96-well plates and were incubated in DMEM with 10% fetal calf serum at 37°C in a humidified, 5% CO2 atmosphere for 18 h. The cell amounts were selected based on the previously conducted experiments and was the 20-50 thousands cells on well of a 96-well plate for MT-4 and Namalva cells. 100 ml DMEM/well with dexamethasone (1 mmol/L), or without for the control cells were added to the samples and incubated 12 hours, then camptothecin (4µg/ml) or without for the control cells were added to the samples and incubated 24 hours. Assays were performed by adding 20μl/well of CellTiter 96→ AQueous One Solution Reagent directly to the culture wells and by incubating for 2 hours and then recording the absorbance at 490nm with a 96-well plate reader. All samples were determined from at least 5 independent experiments.
ChIP and qPCR analysis
ChIP was performed in accordance with the published procedure.Citation29 DNA was cross-linked (1% FA, 10 min) and sheared to a size of 300 base pairs (bp). Approximately 3x106 cells and 10 µg of the antibody were taken for one experiment. After ChIP, the recovered DNA was analyzed by qPCR with MiniOpticon (Bio-Rad). Sequences of the primers used are available upon request. Each point was measured in at least 5 experiments, and the mean value was calculated. The level of intron gene CD3 used as a control.
Statistical analysis
Data are presented as a mean value ± SD. Student two-tiled t-test for the means (paired 2 samples) was used for calculating significance. As pairs we used control series versus sample series affected by dexamethasone, camptothecin or Oct-1 transfection. P-values below 0.05 were considered statistically significant.
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
Author contributions
E. Dukhanina, T. Portseva, E.Pankratova, N. Soshnikova, and A. Stepchenko performed the experiments E. Dukhanina, A. Dukhanin and S. Georgieva have discussed the results and wrote the paper.
Funding
This work was supported by the Russian Science Foundation grant no. 14-15-01032.
References
- Garrett SC, Varney KM, Weber DJ, Bresnick AR. S100A4, a Mediator of Metastasis. J Biol Chem 2006; 281:677-80; PMID:16243835; http://dx.doi.org/10.1074/jbc.R500017200; http://www.ncbi.nlm.nih.gov/pubmed/16243835
- Ismail TM, Fernig DG, Rudland PS, Terry CJ, Wang G, Barraclough R. The basic C-terminal amino acids of calcium-binding protein S100A4 promote metastasis. Carcinogenesis 2008; 9:2259-66; PMID:18784356; http://dx.doi.org/10.1093/carcin/bgn217
- Donato R, Cannon GS, Riuzzi F, Hsu K, Weber DJ, Geczy CL. Functions of S100 Proteins. Curr Mol Med. 2013; 13:24-57; PMID:22834835; http://dx.doi.org/10.2174/156652413804486214
- Semov A, Moreno MJ, Onichtchenko A, Abulrob A, Ball M, Ekiel I, Pietrzynski G, Stanimirovic D, Alakhov V. Metastasis-associated protein S100A4 induces angiogenesis through interaction with Annexin II and accelerated plasmin formation. J Biol Chem. 2005; 280:20833-41; PMID:15788416; http://dx.doi.org/10.1074/jbc.M412653200; http://www.jbc.org/content/280/21/20833.long
- Boye K, Maelandsmo GM. S100A4 and metastasis: a small actor playing many roles. Am J Pathol 2010; 176:528-35; PMID:20019188; http://dx.doi.org/10.2353/ajpath.2010.090526
- Kang J, Gemberling M, Nakamura M, Whitby FG, Handa H, Fairbrother WG, Tantin D. A general mechanism for transcription regulation by Oct1 and Oct4 in response to genotoxic and oxidative stress. Genes Dev 2009; 23:208-222; PMID:19171782; http://dx.doi.org/10.1101/gad.1750709
- Stepchenko AG. Noncanonical Oct-sequences are targets for mouse Oct-2B transcription factor. FEBS Lett 1994; 337:175-8; PMID:8287972; http://dx.doi.org/10.1016/0014-5793(94)80268-8
- Verrijzer CP, Alkema MJ, van Weperen WW, Van Leeuwen HC, Strating MJ, van der Vliet PC. The DNA binding specificity of the bipartite POU domain and its subdomains. EMBO J 1992; 11:4993-5003; PMID:1361172; http://www.ncbi.nlm.nih.gov/pmc/articles/PMC556977/
- Maddox J, Shakya A, South S, Shelton D, Andersen JN, Chidester S, Kang J, Gligorich KM, Jones DA, Spangrude GJ, et al. Transcription factor Oct1 is a somatic and cancer stem cell determinant. PLoS Genet 2012; 8:e1003048; PMID:23144633; http://dx.doi.org/10.1371/journal.pgen.1003048
- Luchina NN, Krivega IV, Pankratova EV. Human Oct-1L isoform has tissue-specific expression pattern similar to Oct-2. Immunol Lett 2003; 85:237-41; PMID:12663137; http://dx.doi.org/10.1016/S0165-2478(02)00179-7
- Dukhanin AS, Romanova EA, Dukhanina EA. Molecular mechanisms of different sensitivity of tumor cells to dexamethasone. Bull Exp Biol Med 2001; 131:88-92; PMID:11329088; http://dx.doi.org/10.1023/A:1017594932500
- Åstrand C, Belikov S, Wrange Ö. Histone acetylation characterizes chromatin presetting by NF1 and Oct1 and enhances glucocorticoid receptor binding to the MMTV promoter. Exp Cell Res 2009; 315:2604-15; PMID:19463811; http://dx.doi.org/10.1016/j.yexcr.2009.05.012
- Qiana Y-H, Qingli Xiaob Q, Hong Chenb H, Xu J. Dexamethasone inhibits camptothecin-induced apoptosis in C6-glioma via activation of stat5/bcl-xl pathway. Biochim Biophys Acta 2009; 1793:764-71; PMID:19339209; http://dx.doi.org/10.1016/j.bbamcr.2009.01.017
- Jaffe J, Hochberg M, Riss J, Hasin T, Reich L, Laskov R. Cloning, sequencing and expression of two isoforms of the murine oct-1 transcription factor. Biochim Biophys Acta 1995; 1261:201-9; PMID:7711063; http://dx.doi.org/10.1016/0167-4781(94)00246-Y
- Belikov S, Gelius B, Wrange Ö. Hormone-induced nucleosome positioning in the MMTV promoter is reversible. EMBO J. 2001; 20(11):2802-11; PMID:11387213; http://dx.doi.org/10.1093/emboj/20.11.2802
- Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer 2006; 6(10):789-802; PMID:NOT_FOUND; http://dx.doi.org/10.1038/nrc1977
- Ge H, Ni S, Wang X, Xu N, Liu Y, Wang X, Wang L, Song D, Song Y, Bai C. Dexamethasone reduces sensitivity to cisplatin by blunting p53-dependent cellular senescence in non-small cell lung cancer. PLoS One 2012; 7(12):e51821; PMID:23272171; http://dx.doi.org/10.1371/journal.pone.0051821
- Ren X1, Siegel R, Kim U, Roeder RG. Direct interactions of OCA-B and TFII-I regulate immunoglobulin heavy-chain gene transcription by facilitating enhancer-promoter communication. Mol Cell 2011; 42(3):342-55; PMID:21549311; http://dx.doi.org/10.1016/j.molcel.2011.04.011
- Chandran UR, Warren BS, Baumann CT, Hager GL, DeFranco DB. The glucocorticoid receptor is tethered to DNA-bound Oct-1 at the mouse gonadotropin-releasing hormone distal negative glucocorticoid response element. J Biol Chem 1999; 274:2372-8; PMID:9891005; http://dx.doi.org/10.1074/jbc.274.4.2372
- Belikov S., Holmqvist P-H., Åstrand C., Wrange O. Nuclear Factor 1 and Octamer Transcription Factor 1 Binding Preset the Chromatin Structure of the Mouse Mammary Tumor Virus Promoter for Hormone Induction. J Biol Chem 2004; 279:49857-67; PMID:15381691; http://dx.doi.org/10.1074/jbc.M409713200
- Sergeev PV, Dukhanin AS. Role of membranotropic effects of glucocorticoids in the realization of their pharmacological activity. Bull Exp Biol Med 2002; 134(3):209-17; PMID:12511983; http://dx.doi.org/10.1023/A:1021462212038
- Kirschke E, Goswami D, Southworth D, Patrick R. Griffin PR, Agard D. Glucocorticoid Receptor Function Regulated by Coordinated Action of the Hsp90 and Hsp70 Chaperone Cycle. Cell 2014; 157:1685-97; PMID:24949977; http://dx.doi.org/10.1016/j.cell.2014.04.038
- Dukhanina EA, Kabanova OD, Lukyanova TI, Shatalov YV, Yashin DV, Romanova EA, Gnuchev NV, Galkin AV, Georgiev GP, Sashchenko LP. Opposite roles of metastasin (S100A4) in two potentially tumoricidal mechanisms involving human lymphocyte protein Tag7 and Hsp70. Proc Natl Acad Sci U S A 2009; 106:13963-7; PMID:19666596; http://dx.doi.org/10.1073/pnas.0900116106
- Zarogoulidis P, Tsakiridis K, Karapantzou C, Lampaki S, Kioumis I, Pitsiou G, Papaiwannou A, Hohenforst-Schmidt W, Huang H, Kesisis G, et al. Use of proteins as biomarkers and their role in carcinogenesis. J Cancer 2015; 6:9-18; PMID:25553084; http://dx.doi.org/10.7150/jca.10560
- Dukhanina EA, Yashin DV, Galkin AV, Sashchenko LP. Unexpected deeds of familiar proteins: Interplay of Hsp70, PGRP-S/Tag7 and S100A4/Mts1 in host vs. cancer combat. Cell Cycle 2010; 9:676-682; PMID:20107319; http://dx.doi.org/10.4161/cc.9.4.10782
- Pankratova EV, Sytina EV, Luchina NN, Krivega IV. The regulation of the Oct-1 gene transcription is mediated by two promoters. Immunol Lett 2003; 88:15-20; PMID:12853155; http://dx.doi.org/10.1016/S0165-2478(03)00026-9
- Pankratova E, Sytina E, Polanovsky O. Autoregulation of Oct-1 gene expression is mediated by two octa-sites in alternative promoter. Biochimie 2006; 88:1323-9; PMID:16716485; http://dx.doi.org/10.1016/j.biochi.2006.04.012
- Dukhanina EA, Lukyanova TI, Romanova EA, Dukhanin AS, Sashchenko LP. Comparative Analysis of Secretion of S100A4 Metastatic Marker by Immune and Tumor Cells. Bull Exp Biol Med 2008; 145:85-7. http://link.springer.com/article/10.1007/s10517-008-0003-z.
- Kopytova DV, Orlova AV, Krasnov AN, Gurskiy DY, Nikolenko JV, Nabirochkina EN, Shidlovskii YV, Georgieva SG. Multifunctional factor ENY2 is associated with the THO complex and promotes its recruitment onto nascent mRNA. Genes Dev 2010; 24:86-96; PMID:20048002; http://dx.doi.org/10.1101/gad.550010
