Aberrant expression and/or activity of chromatin modifying enzymes, such as histone deacetylases (HDACs) have been implicated in many human cancers.Citation1 Consequently, HDAC inhibitors (HDACi) are being developed and tested as cancer therapeutics for multiple malignancies, and have shown efficacy in treating T-cell lymphomas.Citation1 While HDAC inhibition of tumor cells can induce apoptosis and affect cellular processes, including cell cycle and DNA damage/repair, typically resulting in cell death,Citation1,2 the underlying mechanism(s) for how this occurs has remained unclear. Understanding the cellular mechanisms causal to the anticancer activity of HDACi should lead to more efficacious HDAC inhibitors to treat human malignancies, including epithelial-based cancers, such as breast and lung adenocarcinoma. We recently revealed a previously unknown mechanism of HDACi-mediated apoptosis of human breast and lung carcinomas,Citation3 which we also determined is shared in haematopoietic malignancies.Citation4
Studies evaluating mRNA levels reported changes in gene expression following HDACi.Citation1 However, although histone acetylation is linked to transcriptional activation, surprisingly, HDACi did not cause global transcriptional dysregulation, but did alter the mRNA levels of several BCL-2 family members.Citation1 Our data from precision global run-on transcription coupled with massively paralleled sequencing (PRO-seq) showed little difference in the transcription of anti-apoptotic BCL-2 genes after HDACi.Citation4 Consequently, we hypothesized post-transcriptional mechanisms, namely microRNA (miRNA), were likely involved in mediating the gene expression changes following HDACi.
Breast and lung cancers, among others, often present with decreased levels of miRNA, which can be attributed to repression by transcription factors, such as MYC.Citation5 Using multiple pan-class I and selective class I HDACi, we revealed a mechanism whereby MYC represses the tumor suppressor miR-15 and let-7 miRNA families with the help of HDACs to close chromatin and shut down transcription at the miRNA promoters ().Citation3,4 Without MYC present, HDACi had minimal, if any, effect on the expression of the miR-15 and let-7 families. In all cell types analyzed (haematopoietic and non-haematopoietic), HDAC3 appeared to be the predominant HDAC involved in their transcriptional repression.Citation3,4 Similarly, utilizing the same HDAC3-selective compound we evaluated and concurrent with our studies on haematological malignancies,Citation4 myeloid and lymphoid malignancies were reported to be more sensitive to loss of HDAC3 than inhibition of the other class I HDACs.Citation6 Therefore, HDAC3 appears to be primarily responsible for the repression of the miR-15 and let-7 families and the ensuing apoptosis.Citation3,4 Importantly, we determined that HDACi in tumor cells altered the transcriptional program of MYC, switching MYC from a transcriptional repressor to a transcriptional activator of the miR-15 and let-7 families to increase the levels of these tumor suppressor miRNA in breast and lung cancer cells (). Our results indicate that more focused efforts on HDAC3 should improve the efficacy of HDACi therapies for breast and lung carcinoma.
Figure 1. HDAC inhibition re-activates MYC-regulated miRNA mediated-apoptosis. Cellular transformation status dictates whether MYC transcriptionally activates or with HDACs, represses the miR-15 and let-7 families. In normal, non-transformed cells (top), MYC induces these miRNA that then target BCL-2 and BCL-XL, decreasing their expression and triggering apoptosis. This apoptotic mechanism is inactivated in cancer cells (bottom), but re-activated by HDACi (dashed lines).
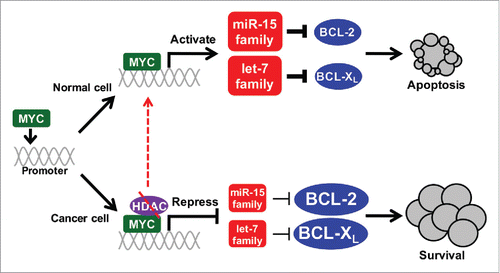
Although the miR-15 and let-7 families were transcriptionally repressed by MYC in cancer cells and re-activated by HDACi, we unexpectedly discovered that their expression was induced by dysregulated MYC in normal, non-transformed cells (). Given that the miR-15 and let-7 families can promote apoptosis by targeting the mRNA of pro-survival genes BCL-2 and BCL-XL, respectively,Citation5 we investigated whether a connection between MYC, these miRNA, and BCL-2 and BCL-XL existed. Our data exposed an unknown link whereby MYC transcriptionally activates the miR-15 and let-7 families in normal cells that directly target and suppress the expression of anti-apoptotic BCL-2 and BCL-XL as a novel tumor suppressive mechanism to counter the hyper-proliferative signals of dysregulated MYC (). Previously, we reported that MYC-mediated apoptosis occurs, in part, by down-regulating BCL-2 and BCL-XL through a mechanism that remained incompletely understood.Citation7 Our recent studies have revealed that the decreased expression of BCL-2 and BCL-XL is due to MYC transcriptionally up-regulating the miR-15 and let-7 families that target BCL-2 and BCL-XL, respectively.Citation3,4 Moreover, our results demonstrate that cellular transformation status dictates whether MYC transcriptionally activates or represses the miR-15 and let-7 families (). We determined that this previously unknown miRNA-mediated mechanism of MYC-induced apoptosis in normal cells is inactivated in breast and lung carcinoma cells through HDAC-mediated alterations, but can be re-activated by HDACi, resulting in tumor cell apoptosis.Citation3 We identified this same mechanism in lymphoid and myeloid cells,Citation4 indicating a universal mechanism that is not cell type specific.
While HDACi has been reported to elicit cell cycle arrest or DNA damage,Citation1,2 the most frequently reported consequence is apoptosis.Citation1 Following HDACi in tumor cells, gene expression profiling showed changes in the expression of BCL-2 family members to favor tumor cell death.Citation1 Our data revealed the molecular mechanism for this is attributed to the MYC-induced increase in transcription of the miR-15 and let-7 families, leading directly to the down-regulation of anti-apoptotic genes BCL-2 and BCL-XL (). Furthermore, HDAC inhibitors have provided clinical benefit in the treatment of some haematological malignancies; however, its impact on the treatment of solid organ cancers, such as breast and lung, remains an active area of investigation. Our recent results contribute critical new data of the molecular events underlying HDACi-induced apoptosis and provide important insight that should aid the development of more efficacious targeted or combination therapies for epithelial-based cancers, as well as for haematological malignancies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- West AC, et al. J Clin Invest 2014; 124:30-9; PMID:24382387; http://dx.doi.org/10.1172/JCI69738
- Conti C, et al. Cancer Res 2010; 70:4470-80; PMID:20460513; http://dx.doi.org/10.1158/0008-5472
- Adams CM, et al. Cell Death Differ 2016; PMID:26915294; http://dx.doi.org/10.1038/cdd.2016.9
- Adams CM, et al. Cancer Res 2016; 76:736-48; PMID:26676759; http://dx.doi.org/10.1158/0008-5472.CAN-15-1751
- Psathas JN, et al. Cold Spring Harb Perspect Med 2014; 4; http://dx.doi.org/10.1101/cshperspect.a014175
- Matthews GM, et al. Blood 2015; 126:2392-403; PMID:26447190; http://dx.doi.org/10.1182/blood-2015-03-632984
- Eischen CM, et al. Mol Cell Biol 2001; 21:5063-70; PMID:11438662; http://dx.doi.org/10.1128/MCB.21.15.5063-5070.2001
