Recent studies have described the cellular components and paracrine factors comprising the haematopoietic stem cells (HSCs) niche in the bone marrow (BM). Various niche-supporting cells (NSPs) in the BM – including osteoblasts (OBs), mesenchymal stromal cells (MSCs), sinusoidal endothelial cells (ECs), CXCL12-abundant reticular (CAR) cells, and macrophages – regulate the self-renewal, dormancy, and differentiation status of HSCs in vivo and ex vivo.Citation1 Although our understanding of the HSC niche in the BM has expanded, many questions remain. CD82 (KAI1) was initially identified as being involved in the T cell activation process.Citation2 A previous study identified DARC (CD234) expressed on ECs as the counterpart molecule to CD82 expressed on cancer cells. Interestingly, CD82-DARC interaction suppresses tumor metastasis.Citation3 While the metastasis suppressor function of CD82 has been extensively explored, other physiological roles of this ubiquitous molecule remain elusive. In our previous studies of prostate cancer, we demonstrated that CD82 expression has been shown to be regulated by Tip60/Pontin coactivators and β-catenin/Reptin corepressors, respectively. Nuclear export of the N-CoR/TAB2/HDAC3 complex by interleukin-1β (IL-1β) induces CD82 expression through the recruitment of the Tip60 complex. Conversely, CD82 expression is suppressed by the β-catenin/reptin complex accompanied by histone deacetylase 3 (HDAC3).Citation4 Next, we reported the existence of hypoxia responsive element in Cd82 promoter region and upregulation of CD82 expression in ischemic cardiac tissues. The study indicates that CD82 may play an important role in hypoxic stem cell niche such as BM.
We recently reported that cell cycle status of CD82-positive long-term repopulating HSCs (LT-HSCs) is regulated by BM macrophages ().Citation2 At the onset of that study, our 2 main questions were: first, what is the exclusive surface marker of LT-HSCs that distinguishes them from short-term repopulating HSCs (ST-HSCs) or progenitors; and second, what is the main stem cell niche player that supports LT-HSC quiescence? Consistent with previous reports, CD82 was broadly expressed in various cell types in murine BM. However, among stem-progenitor cell population, Cd82 was expressed almost exclusively on LT-HSCs; little was detected on ST-HSCs and MPPs. Moreover, only LT-HSCs expressed CD82 on the cell surface. Of note, CD82 maintained/promoted LT-HSC quiescence by activating the TGF-β/SMAD pathway and CDK inhibitors (p21, p27 and p57) that induce G0/G1 arrest. We then explored the possibility that Cd82 knockout NSPs may influence cell cycle status of LT-HSCs. Interestingly, while Cd82 deficiency of LT-HSCs allowed the cells to switch from G0 to G1 phase, the absence of CD82 on MSCs accelerated the G1-to-S progression in LT-HSCs. Further study is warranted to determine the precise mechanism through which CD82 expressed on MSCs promotes LT-HSC dormancy. We also identified DARC expressed on macrophages, as a binding partner for CD82 on LT-HSCs in the BM. Upon BM macrophage ablation, LT-HSCs lost direct contact with DARC on macrophages, resulting in ubiquitination and endocytosis of cell surface CD82 molecules. Decreased surface CD82 level of LT-HSCs led to cell cycle entry and proliferation. Conversely, macrophagic DARC or recombinant DARC protein blocked the endocytosis of CD82 on the surface of LT-HSCs (). Given that CD82 is not only a surface marker but also maintains LT-HSC quiescence thereby affecting BM reconstituting capacity, elucidating how CD82 expression is restricted to LT-HSCs through a precise underlying molecular mechanism is of vital interest. Recently, macrophages have been identified as positive regulators for maintenance and retention of HSC niches.Citation1,5 Our finding that DARC+ macrophages show a functional overlap with COX-2high macrophages corroborates the significance of macrophages in governing HSC quiescence. Further study is required to examine whether the CD82-DARC axis exists in other tissues such as blood vessels, muscle, or heart with an emphasis on tissue-resident stem cells. Considering various functions of macrophages in the tumor,Citation6 it would be also interesting to see whether CD82/DARC interaction exists between cancer stem cells and macrophages in their niches. The finding that 33% of LT-HSCs from Cd82−/− mouse are dormant suggests the importance of other unknown factors and NSPs contributing to LT-HSC quiescence. the differences between DARC+ and DARC– macrophages and crosstalk among HSCs, OBs, MSCs, ECs, and other NSPs will improve our understanding of the bona fide BM stem cell niche.
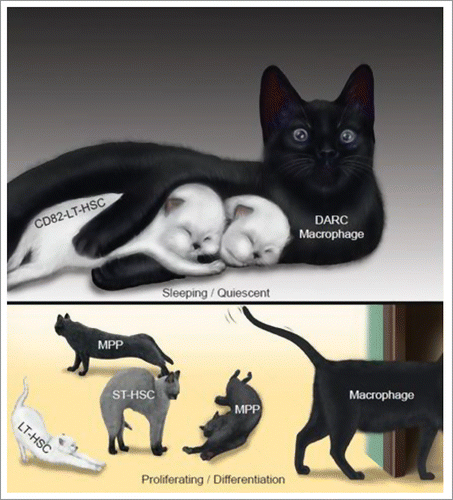
Figure 1. CD82(KAI1) on LT-HSCs interacts with its binding partner DARC expressed on macrophages, to maintain LT-HSC quiescence. This is a metaphorical image depicting the relationship of DARC+ macrophages (black-furred mother cat), CD82+ LT-HSCs (white-furred kittens) and other haematopoietic stem-progenitors (gray-furred kittens). When the mother holds the kittens in her forelegs in the dark (DARC), they fall asleep. During the day (the absence of macrophagic DARC), the mother lets her kittens out; they then wake up and undergo proliferation and differentiation.
One application of our study that we persue now is the development of strategies to eradicate leukemic stem cells (LSC). The most common cause of treatment failure in leukemia patients is relapse due to dormant LSCs that are not eliminated by anti-proliferation chemotherapy. Therefore, 2-step therapy has been proposed to remove LSCs: 1) to bring LSCs into cell cycle; and 2) to destroy cycling LSCs with chemotherapy.Citation7 This, in combination with previous strategies to awaken the quiescent HSCs using granulocyte colony-stimulating factor, interferon-α and arsenic trioxide, may enable the complete elimination of LSCs. Finally, we hope that further development of our study will allow us to improve leukemia treatment by awakening not only “good” stem cells to keep beneficial stem cell functions, but also “bad” stem cells (e.g. cancer stem cells) in order to increase the sensitivity to treatment and avoid relapse. The differential regulation of awakening or dormancy between cancer versus normal stem cells may be possible when we fully dissect the underlying biology of the CD82-DARC axis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Hyo-Soo Kim is supported by the Innovative Research Institute for Cell Therapy (A062260) and Korea Health Technology R&D Project (HI14C1277) through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health and Welfare (MHW). Sung Hee Baek is supported by a grant from the Creative Research Initiatives Program (2009-0081563) of the National Research Foundation (NRF) funded by the Korean government (MSIP).
References
- Ehninger A. et al. J Exp Med 2011; 208:421-8; PMID:21402747; http://dx.doi.org/10.1084/jem.20110132
- Hur J. et al. Cell Stem Cell 2016; 18:508-21; PMID:26996598; http://dx.doi.org/10.1016/j.stem.2016.01.013
- Bandyopadhyay S. et al. Nat Med 2006; 12:933-8; PMID:16862154; http://dx.doi.org/10.1038/nm1444
- Kim JH. et al. Nature 2005; 434:921-6; PMID:15829968; http://dx.doi.org/10.1038/nature03452
- Hur J. et al. Cell Res 2011; 21:987-90; PMID:21483451; http://dx.doi.org/10.1038/cr.2011.69
- Park J. et al. Cell Rep 2015; 10:1614-25; http://dx.doi.org/10.1016/j.celrep.2015.02.024
- Trumpp A. et al. Immunology 2010; 10:201-9; PMID:20182459; http://dx.doi.org/10.1038/nri2726
