Defects in chromosome segregation machinery give rise to generation of aneuploid cells containing abnormal number of chromosomes. The chromosomal passenger complex (CPC) composed of 4 core subunits, Aurora B kinase, INCENP, Survivin and Borealin/Dasra ensures faithful chromosome segregation primarily by releasing incorrect kinetochore-microtubule attachments. Earlier studies have found that a partial inhibition of Aurora B activity by a low-dose of inhibitor treatment already induces chromosome segregation errors, whereas more potent inhibition is needed to affect Aurora B-mediated histone H3 phosphorylation, chromosome assembly and movement and cytokinesis.Citation1 These results have implied that fidelity of chromosome segregation depends on full activity of Aurora B. Although cancer cells, in general, have elevated rates of chromosome missegregation, molecular basis underlying such pathological conditions has been a longstanding question.
The heterochromatin protein 1 (HP1) associates with CPC through INCENP and thereby is recruited to centromeres in mitosis.Citation2,3 The significance of this HP1-CPC association was unclear until recently, when an allosteric effect of HP1 on Aurora B was found.Citation4 HP1 doubled the substrate-processing rate of Aurora B in vitro, and this enhancement of the kinase activity is required for Aurora B to phosphorylate kinetochore substrates in cells. Remarkably, the fraction of HP1-bound CPC was significantly decreased in all the cancer cell lines tested, which led to the conclusion that insufficient Aurora B activity is a widespread feature of cancers. A corollary of these findings was that HP1-mediated full Aurora B activity supports the fail-safe chromosome segregation and indeed, disruption of HP1 binding to the CPC in non-transformed cells increased the rate of chromosome missegregation, without detectably affecting other mitotic events regulated by Aurora B.Citation4
Deprivation of HP1 from the CPC lowered Aurora B activity and perturbed the phosphorylation of kinetochore substrates, but it also impaired the enrichment of Aurora B at centromeres.Citation4 These results are consistent with the notion that CPC loading onto centromeres is regulated by multiple positive feedback loops, involving Aurora B activity itself.Citation5 For instance, Aurora B enhances centromeric accumulation of the CPC by regulating Haspin kinase-mediated histone H3-phospho-Thr3, which in turn recruits the CPC at centromeres. Moreover, because Aurora B is known to promote chromatin association of condensin I, which confers physical stability to centromeres,Citation6 accumulation of Aurora B activity at centromeres must be important to maintain the structural integrity at that site. Thus the “scaffolds” for CPC may become unstructured without an allosteric effect of HP1. Based on these observations, a reasonable view would be that the proper centromeric localization and function of the CPC are controlled by self-regulating mechanisms of Aurora B (). The finding that non-transformed cells have a robust mechanism to correct attachment errors by increasing Aurora B recruitment to misaligned chromosomesCitation7 may underscore the importance of this system-level control.
Figure 1. Positive feedback loops underlying regulation of the CPC. Proper centromeric localization and function of the CPC is regulated by multiple positive feedback loops based on Aurora B activity. HP1-assisted Aurora B full activity in non-transformed cells forms more robust feedback loops than that in cancer cells. Cancer cells have system-level deficiencies in Aurora B control by incomplete HP1 binding.
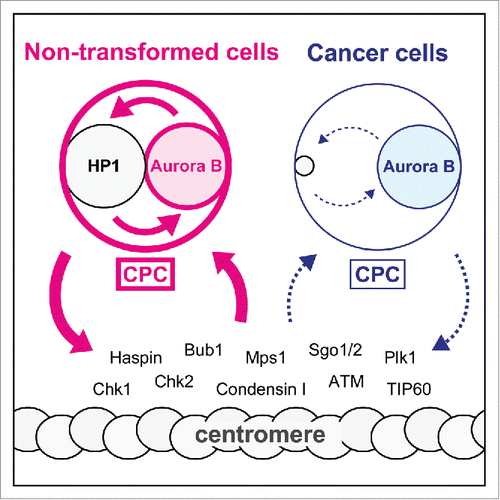
In a wide range of cancer cells, defined localization of Aurora B at centromeres is noticeably impaired to various extents, and it is instead remained undetached from chromosome arms (unpublished observations, Abe Y and Hirota T). In light of positive feedback loops controlling localization of Aurora B, this ill-defined centromeric localization can be reasonably explained by the finding that the activity of Aurora B is low by decreased HP1 binding in cancer cells.Citation4 It is noteworthy to point out the interlinked positive feedback loop between HP1 and the CPC. As the association of HP1 with the CPC is stabilized by Aurora B-mediated phosphorylation of HP1 through an incompletely understood mechanism,Citation4 the stable binding of HP1 to the CPC will keep the activity of Aurora B in a high level state (). Thus, the existence of system level controls allows us to predict that the activity of Aurora B is settled down to a steady state with lower levels, which renders cancer cells predisposed to chromosomes segregation errors in mitosis. The results that over-expression of HP1 did not facilitate formation of HP1-CPC at allCitation4 well supports the notion that cancer cells suffer from the system-level deficiency of Aurora B, the condition reflected by an inherent limitation of HP1-binding ability.
What limits the binding capacity of HP1 to the CPC in cancer cells is an important following question. It cannot be explained simply by the change in the protein expression levels of HP1.Citation4 Genomic imbalance caused by gain or loss of chromosomes would disturb proteome composition. Thus, to maintain proteostasis, aneuploid cells may greatly rely on the cellular protein quality control system, including chaperone, autophagy or proteasome systems. Chaperone protein families support folding of aberrant proteins as well as newly synthesized polypeptides and, also assembly of multi-protein complexes. Formation of HP1-CPC may depend on a chaperone protein, and hence HP1 binding may be reduced in cancer cells in which chaperones are in short supply. Importantly, malignant transformation of chromosomally stable, non-transformed cells resulted in an elevated rate of chromosome missegregation, and of note, this was associated with an apparent reduction of the HP1-bound CPC.Citation4 Thus, transformation targets the Aurora B system and studying the process of transformation should provide a unique clue to address pathological changes in centromere-kinetochore components.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol 2006; 16:1711-8; PMID:16950108; http://dx.doi.org/10.1016/j.cub.2006.07.022
- Ainsztein AM, Kandels-Lewis SE, Mackay AM, Earnshaw WC. INCENP centromere and spindle targeting: Identification of essential conserved motifs and involvement of heterochromatin protein HP1. J Cell Biol 1998; 143:1763-74; PMID:9864353; http://dx.doi.org/10.1083/jcb.143.7.1763
- Kang J, Chaudhary J, Dong H, Kim S, Brautigam CA, Yu H. Mitotic centromeric targeting of HP1 and its binding to Sgo1 are dispensable for sister-chromatid cohesion in human cells. Mol Biol Cell 2011; 22:1181-90; PMID:21346195; http://dx.doi.org/10.1091/mbc.E11-01-0009
- Abe Y, Sako K, Takagaki K, Hirayama Y, Uchida KSK, Herman JA, DeLuca JG, Hirota T. HP1-assisted aurora B kinase activity prevents chromosome segregation errors. Dev Cell 2016; 36:487-97; PMID:26954544; http://dx.doi.org/10.1016/j.devcel.2016.02.008
- van der Horst A, Lens SMA. Cell division: control of the chromosomal passenger complex in time and space. Chromosoma 2014; 123:25-42; PMID:24091645; http://dx.doi.org/10.1007/s00412-013-0437-6
- Lipp JJ, Hirota T, Poser I, Peters J-M. Aurora B controls the association of condensin I but not condensin II with mitotic chromosomes. J Cell Sci 2007; 120:1245-55; PMID:17356064; http://dx.doi.org/10.1242/jcs.03425
- Salimian KJ, Ballister ER, Smoak EM, Wood S, Panchenko T, Lampson MA, Black BE. Feedback control in sensing chromosome biorientation by the aurora B kinase. Curr Biol 2011; 21:1158-65; PMID:21723127; http://dx.doi.org/10.1016/j.cub.2011.06.015
