Cell growth and death are tightly regulated mechanisms that, in concert, facilitate tissue formation and integrity. Disruption in either pathway is a well-described hallmark that cancer cells exploit to survive noxious stimuli such as DNA damage, Reactive Oxygen Species (ROS), Fas-Fas-Ligand interaction or loss of cell-cell adhesion.Citation1 Thus, many chemotherapeutics aim to disrupt survival and drive cells down a Caspase 8 mediated apoptotic pathway. Necroptosis is a more recently described mechanism of cell death that denotes coordinated cellular necrosis, leading to pore formation in the cell membrane and the release of cellular components. Evolutionarily, necroptosis can be considered a second line of defense against viruses, which enter cells and inhibit cellular demise via Caspase 8-mediated apoptosis. In the presence of dysfunctional Caspase 8, receptor interacting kinase (RIP) 1 and 3 associate and assemble the necrosome, which in turn recruits mixed lineage kinase domain-like protein (MLKL) leading to necroptosis and the release of danger signals.Citation2
Our recent work shows that the components of the necrosome are highly expressed in human and murine pancreatic ductal adenocarcinoma (PDA).Citation3 Expression was further inducible by the chemotherapeutic agent Gemcitabine. To investigate the role of necroptosis in PDA progression, we deleted RIP3 in pancreatic cancer cells. In accordance with our hypothesis, RIP3 deletion resulted in a more aggressive oncogenic phenotype in vitro. However, contrary to these observations RIP3 deletion in vivo resulted in marked protection against PDA including a significant survival benefit. RIP1 inhibition was similarly protective. We found that targeting the necrosome led to a more immunogenic inflammatory infiltrate as evidenced by an increase in tumor infiltrating CD8+ T cells and Th1-polarized CD4+ T cells, B cells, as well as a reduction in myeloid derived suppressor cells (MDSCs) and tumor associated macrophages (TAMs). Furthermore, TAMs exhibited a shift toward an M1-like immunogenic phenotype. Interestingly, pro-tumorigenic effects of necroptosis seemed to be specific to the pancreatic tumor microenvironment (TME), as neither subcutaneously implanted PDA nor B16 melanoma cells exhibited altered tumor growth in the context of RIP3 deletion.
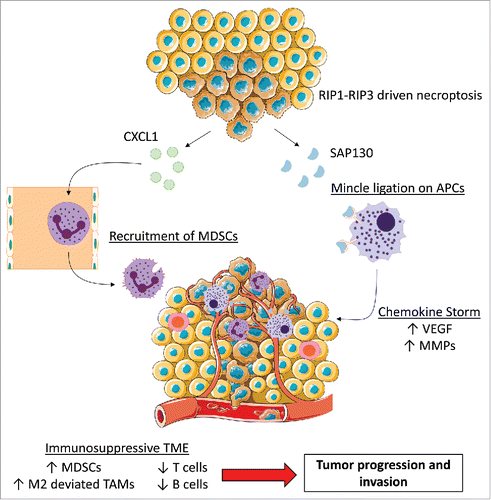
We discovered that CXCL1, a potent chemoattractant for myeloid cells, was highly expressed in a RIP3 dependent manner. We postulated that CXCL1 could mediate the pro-tumorigenic immune suppression associated with RIP3 signaling by mobilizing myeloid cells. CXCL1 blockade protected against PDA, reduced myeloid cell infiltration, and increased peri-tumoral T cells. However, CXCL1 overexpression alone could not account for the entire immunosuppressive phenotype associated with intact necroptosis signaling in PDA.
Since necroptosis produces danger signals to the surrounding environment, we postulated that the release of damage associated molecular patterns (DAMPs) by necroptotic cells within the TME triggers immune-suppressive inflammation. Mincle is a C type lectin receptor involved in fungal immunity and has recently been implicated in promoting sterile inflammation by ligating SAP130, a subunit of the histone deacetylase complex, which is released by necrotic cells.Citation4 We found that Mincle and its associated signaling intermediates were highly expressed on antigen presenting cells (APCs) and Mincle co-associated with SAP130 in the PDA TME. Further emphasizing the role of necroptosis, SAP130 levels and Mincle signaling were reduced in RIP3−/− tumors. Moreover, Mincle deletion was protective against PDA, extended survival, and directly phenocopied the immunogenic infiltrate associated with RIP3 deletion. In contrast, Mincle ligation led to tumor progression, promoted MDSC infiltration and M2-polarization of TAMs and induced adaptive immune suppression. Cellular depletion experiments revealed that TAMs promote tumorigenesis in PDA; however, they lose their immune-suppressive effects when RIP3 or Mincle are deleted. As such, T cells, which do not protect against PDA progression in hosts with intact RIP3 or Mincle signaling, are reprogrammed into potent mediators of anti-tumor immunity in the absence of RIP3 or Mincle. Our work describes parallel axes of necroptosis-induced CXCL1 and Mincle signaling that promote macrophage-induced adaptive immune suppression and thereby enable pancreatic cancer progression (). Each of these axes represents a novel target for experimental therapeutics.
Figure 1. RIP1-RIP3 driven necroptosis initiates 2 parallel mechanisms to induce adaptive immune-suppression. Release of CXCL1 by necroptotic cells acts as a potent recruiter of MDSCs. Mincle expressed by antigen presenting cells (APCs) can detect SAP130 and further drive an immunosuppressive cytokine milieu. Both axes supply the tumor with growth factors, thereby supporting PDA progression. (Some images adapted from Servier Medical Art (www.servier.com) under the Creative Commons License.)
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Ouyang L, Shi Z, Zhao S, Wang F-T, Zhou T-T, Liu B, Bao J-K. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell proliferation 2012; 45:487-498; PMID:23030059; http://dx.doi.org/10.1111/j.1365-2184.2012.00845.x
- Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nature reviews. Molecular cell biology 2014; 15:135-147; PMID:24452471; http://dx.doi.org/10.1038/nrm3737
- Seifert L, Werba G, Tiwari S, Ly NNG, Alothman S, Alqunaibit D, Avanzi A, Barilla R, Daley D, Greco SH, et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature 2016; 532:245-249; PMID:27049944; http://dx.doi.org/10.1038/nature17403
- Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nature immunology 2008; 9:1179-1188; PMID:18776906; http://dx.doi.org/10.1038/ni.1651
