Cancer metastasis accounts for approximately 90% of cancer-related deaths yet available therapeutics are often not effective at eliminating metastatic cells. Metastasis is an inherently inefficient process as cells are exposed to foreign extracellular stimuli that can engender cell death pathways. Successful navigation of the metastatic cascade by cancer cells requires these cells to inhibit anoikis, a caspase-dependent cell death program that is triggered by detachment from the extracellular matrix (ECM).Citation1 In addition, cancer cells must overcome anoikis-independent metabolic deficiencies that compromise cellular viability.Citation1,2 In particular, ECM-detachment induced metabolic deficiencies often involve insufficient uptake of glucose that consequently leads to diminished ATP production. Studies examining the molecular mechanisms utilized by cancer cells to evade anoikis have discovered that cancer cells employ a multitude of distinct strategies to successfully block the induction of anoikis.Citation3,4 However, the signal transduction employed by cancer cells to ameliorate ECM-detachment induced deficiencies in ATP generation remains poorly characterized. Recently, we have uncovered a novel molecular mechanism downstream of oncogenic Ras that involves the utilization of divergent downstream effector molecules to simultaneously inhibit anoikis and promote productive metabolism during ECM-detachment (summarized in ).Citation5 Here, we discuss these findings in more detail and how this information may be exploited to design novel therapeutic strategies that can eliminate metastatic cancer cells.
Figure 1. A model for Ras-mediated ATP generation and anoikis evasion in ECM-detached cells. Upon detachment from the ECM (black), normal epithelial cells (yellow) undergo anoikis and are faced with metabolic deficiencies that compromise their viability (far right). However, addition of oncogenic Ras (blue) can cause cells to overcome anoikis and promote productive ATP generation through the exhibited mechanism.
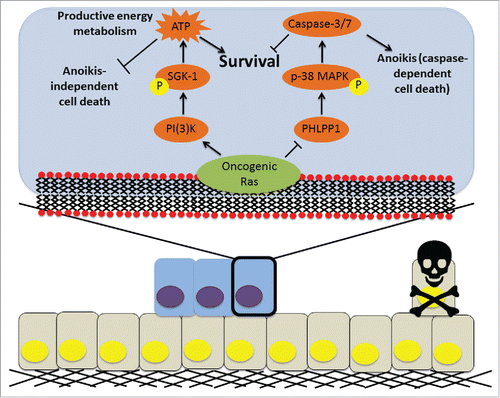
Based on our previous studies examining ErbB2 signaling during ECM-detachment,Citation2 we postulated that signal transduction emanating from activated oncogenes might be a broadly effective strategy utilized by ECM-detached cancer cells to promote ATP generation. In support of this, we discovered that constitutive activation of Ras (either H-Ras and K-Ras) has the capacity to promote ATP generation and subsequent survival during ECM-detachment by increasing glucose uptake.Citation5 This Ras-mediated promotion of ATP generation is mediated by phosphatidylinositol 3-kinase (PI3K), but surprisingly, does not require Akt activation. Instead, we find that the kinase activity of serum and glucocorticoid kinase-1 (SGK-1) is necessary for Ras-mediated glucose uptake, ATP generation, and survival during ECM-detachment. More specifically, using RNAi based approaches to inhibit SGK-1, we find that loss of SGK-1 activity in colon cancer cells containing activating Ras mutations compromises anchorage-independent growth in soft agar. Furthermore, constitutive activation of SGK-1 is sufficient to promote ATP generation, glucose uptake, and survival of ECM-detached cells in 3-dimensional models of mammary acini. In further support of a critical role for SGK-1 downstream of Ras, we find that enhanced SGK-1 activity correlates with elevated quantities of oncogenic Ras in colorectal cancer patients.
Given that SGK-1-mediated ATP generation and survival during ECM-detachment occurs independently of anoikis regulation, we reasoned that Ras was using a distinct signaling pathway to overcome anoikis. Interestingly, in ECM-detached cells with oncogenic Ras, Akt phosphorylation (at Ser473) does not diminish when PI3K is inhibited suggesting that phosphatase activity against Akt may be impaired due to Ras activation. PH domain and leucine rich protein phosphatase 1 (PHLPP1) is a known antagonist of Ser473 on Akt,Citation6 and indeed, oncogenic Ras negatively modulates PHLPP1 protein levels during ECM-detachment. Rescuing PHLPP1 levels in ECM-detached cells with oncogenic Ras sensitizes these cells to anoikis and restricts anchorage-independent growth in soft agar. Furthermore, elimination of PHLPP1 expression is sufficient to hinder anoikis induction and to promote the survival of ECM-detached cells in the lumen of organotypic models of mammary acinus development. Interestingly, it is not the ability of PHLPP1 to dephosphorylate Akt that promotes anoikis induction. Rather, anoikis induction by PHLPP1 is mediated by downstream activation of the p38 MAPK pathway. In support of our data demonstrating that Ras impedes PHLPP1-mediated p38 MAPK activation and ensuing anoikis, we find a negative correlation between levels of oncogenic Ras and the magnitude of p38 MAPK activation in patients with colorectal carcinomas.
In aggregate, our data suggest that oncogenic Ras utilizes divergent downstream effectors to overcome ECM-detachment-induced ATP deficiencies and anoikis induction.Citation5 In line with this premise, simultaneous loss of SGK-1 activity and gain of PHLPP1 is incredibly effective at restraining anchorage independent growth in soft agar.
Our study adds to the growing body of literature suggesting that Akt is not the only prominent effector downstream of PI3K to regulate oncogenic cellular behavior. Furthermore, our data suggest that downstream signaling from oncogenic Ras operates in stark contrast to ErbB2 in its mechanisms for promoting ATP generation during ECM-detachment.Citation2 Given this, it seems reasonable to conclude that cancer cells may be endowed with the capacity to employ diverse strategies to remedy defects in ATP generation during ECM-detachment. Additionally, our data indicate that a single oncogenic insult (hyperactivation of Ras) can promote the survival of ECM-detached cells through stimulation of ATP generation and concurrent inhibition of anoikis. Therefore, an effective strategy to eliminate ECM detached cells with oncogenic Ras mutations needs to involve anoikis induction and mitigate ATP generation. One approach might involve simultaneous inhibition of SGK-1 (which has recently shown promise in triple negative breast cancerCitation7) and restoration of PHLPP1 and p38 MAPK mediated induction of anoikis. Future studies aimed at assessing the efficacy of such a strategy will be critical for ultimately developing novel therapeutics that impede metastasis by targeting and eliminating ECM-detached cancer cells.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Buchheit CL, et al. Nat Rev Cancer 2014; 14:632-41; PMID:25098270; http://dx.doi.org/10.1038/nrc3789
- Schafer ZT, et al. Nature 2009; 461:109-13; PMID:19693011; http://dx.doi.org/10.1038/nature08268
- Rayavarapu RR, et al. J Biol Chem 2015; 290:8722-33; PMID:25681438; http://dx.doi.org/10.1074/jbc.M114.612754
- Weigel KJ JA, et al. Mol Cancer Res 2014; 12:855-66; PMID:24803643; http://dx.doi.org/10.1158/1541-7786.MCR-14-0090
- Mason JA, et al. Cell Death Differ 2016 Feb 26; PMID:26915296; http://dx.doi.org/10.1038/cdd.2016.15
- Gao T, et al. Mol Cell 2005; 18:13-24; PMID:15808505; http://dx.doi.org/10.1016/j.molcel.2005.03.008
- Skor MN, et al. Clin Cancer Res 2013; 19:6163-72; PMID:24016618; http://dx.doi.org/10.1158/1078-0432.CCR-12-3826
