KEYWORDS:
Germ cells are uniquely programmed cell lineage that can transfer genetic and epigenetic information to subsequent generations. Similar to somatic cells, germ cells undergo mitosis for substantial durations to increase their numbers. However, germ cells switch their mode of cell division from mitosis to meiosis to convert to haploid cells at appropriate time points; female germ cells undergo meiosis in the genital ridge at midgestation, but male germ cells initiate this switch after birth in the testes of mammals. Although it is known that retinoic acid is crucially involved in this transition, the molecular mechanisms governing the switch remain largely obscure. However, our recent study revealed MAX as a negative regulator of meiosis, suppressing ectopic and precocious meiotic onset in embryonic stem cells (ESCs) and spermatogonial stem cells (SSCs), respectively.Citation1
Before reaching this conclusion, we first demonstrated that Max expression ablation in ESCs not only activates expression of meiosis-related genes, but also induces cytological changes reminiscent of germ cells at leptotene and zygotene stages of meiosis by immunocytochemical analysis of SYCP3, a major component of the synaptonemal complex.Citation2 We also showed that these cytological changes are dependent on the retinoic acid-STRA8 axis, underscoring that these changes faithfully recapitulate bona fide meiotic processes. However, intriguingly, we found that Max expression-ablated ESCs appear to directly convert to meiosis-like cells without passing through the primordial germ cell state. Subsequently, we demonstrated that the Max gene shows a transient but significant decline in its expression level during the early stage of meiosis in both male and female germ cells, implying that downregulation of Max expression levels is a physiologically required step for meiosis. Finally, we found that forced reduction of Max expression in SSCs induces meiotic entry with much greater efficiency compared with that observed in ESCs.
MAX is generally known as an obligated partner protein of MYC that functions as a transcription factor.Citation3 Indeed, MYC by itself has almost no intrinsic DNA-binding activity, and MAX confers that activity on MYC. MYC/MAX transcription complexes activate transcription of numerous genes, especially those encoding positive regulators of the cell cycle, such as cyclin D, leading to acceleration of cell proliferation.Citation4 However, it has recently been shown that MAX is also a subunit of atypical polycomb complex 1 (PRC1.6),Citation5 and we now know that the Max expression ablation-mediated meiotic onset in ESCs and SSCs reflects disruption of the transcriptional repressing function of PRC1.6.
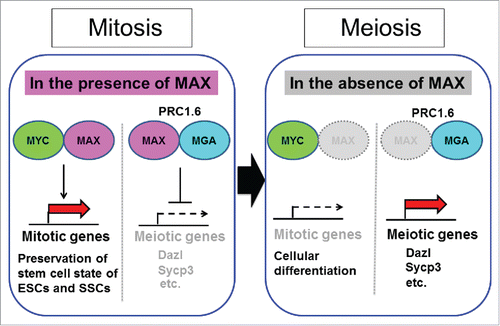
MAX is a unique component of PRC1.6 that not present in any of the other 5 PRC1 complex subtypes (PRC1.1–5).Citation5 PRC1.6 consists of at least 13 subunits including MAX. Furthermore, similar to Max expression deficiency, knockdown of genes encoding other components of this complex, such as L3MBTL2, augments expression levels of meiosis-related genes in ESCs. However, the magnitude of alteration in the expression levels accompanied by Max expression deficiency is much more conspicuous than that resulting from knockdown of genes encoding other components of the PRC1.6 complex. Notably, our study demonstrating the consequence of Max expression deficiency is the only report showing that transcriptional activation of meiotic genes is accompanied by cytological changes reminiscent of meiosis.Citation1 One obvious possibility explaining these conspicuous phenotypes associated with Max expression ablation, but not the loss of expression of genes corresponding to other components of the PRC1.6 complex, is the dependence of the complex on MAX for its binding to DNA. However, we assume that loss of MYC activity associated with the loss of MAX, but not the loss of other genes, may also be related to the amplification of the levels of meiotic changes in ESCs and SSCs. MYC plays crucial roles in preserving the stem cell state of both cell types by activating several genes directly linked to cell proliferation,Citation6,7 which is apparently contradictory phenomenon against meiosis. Consistent with this notion, our results demonstrated that induction of meiosis-like changes by disruption of the PRC1.6 complex through Max expression ablation becomes much less prominent if MYC activity is retained with the aid of mutant MYC/MAX pairs.Citation1 depicts our idea that consequences of Max expression ablation in ESCs and SSCs are 2-fold. One is de-repression of meiotic genes caused by disruption of the PRC1.6 complex that is directly linked to meiosis. The other is disruption of the stem cell state due to the loss of MYC activity that may also indirectly support meiosis.
Figure 1. Model of possible inverse regulation of the expression of mitotic and meiotic genes by controlling Max expression alone. Upon a decline in Max expression levels by either artificial or physiologically in ESCs and SSCs, mitotic and meiotic genes reduce and enhance their expression levels, respectively, through the loss of MYC/MAX complex-dependent transcriptional activation and liberation from the PRC1.6-dependent repression mechanism.
In summary, we provide solid evidence about the involvement of MAX in the transition from mitosis to meiosis. We hope that identification of a new player in this transition will eventually lead to complete understanding of this process at the molecular level. We also hope to eventually understand the molecular basis of germline tumor formation, as these tumors might be explained by the failure of germ cells to transition from mitosis to meiosis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
A.O. is the recipient of grants from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (16H01220, 16H05143 and 16K15223).
References
- Suzuki A, et al. Nat Commun 2016; 7:11056; PMID:27025988; http://dx.doi.org/10.1038/ncomms11056
- Heyting C. Curr Opin Cell Biol 1996; 8:389-96; PMID:8743892; http://dx.doi.org/10.1016/S0955-0674(96)80015-9
- Blackwood EM, Eisenman RN. Science 1991; 251:1211-7; PMID: 2006410
- Baudino TA, Cleveland JL. Mol Cell Biol 2001; 21:691-702; PMID: 11154257; http://dx.doi.org/10.1128/MCB.21.3.691-702.2001
- Gao Z, et al. Mol Cell 2012; 45:344-56; PMID: 22325352; http://dx.doi.org/10.1016/j.molcel.2011.04.004
- Hishida T, et al. Cell Stem Cell 2011; 9:37-49; PMID:21726832; http://dx.doi.org/10.1016/j.stem.2011.04.020
- Kanatsu-Shinohara M, et al. Proc Natl Acad Sci U S A 2014; 111:8826-31; PMID: 24879440; http://dx.doi.org/10.1073/pnas.1401837111
