In order to form functional organs, it is essential to regulate the proliferation rate of progenitor cells as well as their differentiation to generate an ordered series of cell types. Such regulation defines the size and diversity of the resulting cells. How progenitor cells control the timing of their proliferation and the generation of a particular type of cellular progeny at the appropriate time is a fundamental question in developmental biology.Citation1
During mammalian cerebral development, self-renewing neural progenitor cells, also called apical progenitor cells or radial glial cells, undergo a change from proliferative to neurogenic division, and change their laminar fate potential from early-born lower-layer neurons to late-born upper-layer neurons. Many factors are reportedly involved in these transitions;Citation1,2 however, the timing mechanism that counts and controls the progression of these transitions remains largely unknown. Clonal culture of neural progenitor cells has revealed that the mechanism controlling sequential generation of neuron types is encoded within the progenitor cells’ lineages.Citation3 Since neural progenitor cells undergo a stereotypical number of divisions during neocortical development, it has been postulated that transition of temporal character of neural progenitor cells is coupled with cell-cycle progression or cytokinesis.Citation1 Nevertheless, is the cell cycle truly an intrinsic timer that counts and controls temporal progression? How is the timing mechanism associated with the environmental cues?
We have recently addressed these questions using genome-wide transcriptome profiling of single cells. Through statistical analysis of the single cell microarray data sets,Citation4,5 we identified a set of genes that function as markers of temporal change of neural progenitors from early to late neurogenic stages. Some of these ‘temporal-axis’ genes are involved in cell proliferation, consistent with the fact that the majority of neural progenitor cells switch from proliferative to neurogenic division. Notably, major changes in temporal-axis genes are inherited by differentiating intermediate progenitor cells from undifferentiated neural progenitor cells. This situation resembles the case of temporal identity genes in Drosophila neural progenitor cells, which are expressed in both neuroblasts and ganglion mother cells, indicating that temporal-axis genes satisfy at least one of the criteria that defines temporal identity genes.Citation2
We subsequently determined whether cell-cycle progression is required to alter temporal gene expression in neural progenitors. Overexpression of CDK inhibitor by in vivo electroporation arrests the cell-cycle progression of neural progenitor cells; however, it also leads to precocious differentiation of the progenitor cells. Therefore, we simultaneously overexpressed the Cdk4 inhibitor Cdkn2c (p18) and the intracellular domain of Notch1 (NICD) because NICD maintains self-renewal potential. Two to 4 d after in vivo electroporation, we harvested the cell cycle–arrested neural progenitor cells expressing p18 and NICD, and determined their gene expression profiles. Surprisingly, we found that temporal gene expression changed even in cell cycle–arrested cells as is observed during normal cortical development. Furthermore, transient cell-cycle arrest in vivo does not interfere with the normally occurring laminar fate transition of neural progenitor cells from deep layers to upper layers (), indicating that cortical progenitors do not need to progress through the cell cycle to promote temporal progression of laminar fate potential; i.e., cell cycle–arrested neural progenitor cells still know the right time.
Figure 1. Cell-cycle progression is not necessary for transitions in temporal gene expression and laminar fate potential of neural progenitor cells during mammalian cerebral development. Transient cell-cycle arrest by in vivo p18/NICD co-expression and Cre-mediated recombination does not interfere with the laminar fate transition of neural progenitor cells from deep layers to upper layers.
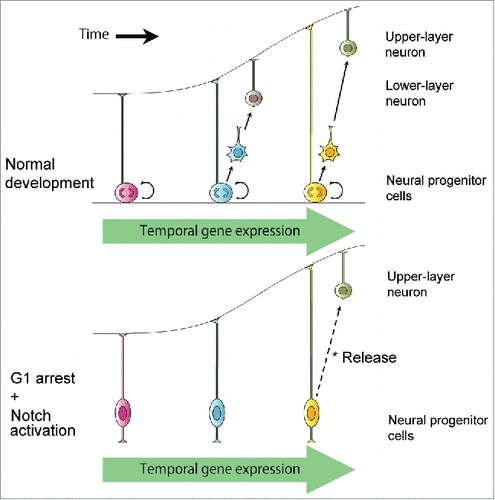
It is therefore unlikely that cell division works as a timer in neural progenitor cells to determine the timing of the shift in their temporal identity. This situation is similar to that in Drosophila embryonic neuroblasts, which sequentially express 4 genes, hunchback, Kruppel, pdm1, and caster, in that order. Although the hunchback–Kruppel transition requires neuroblast cytokinesis, the Kruppel–pdm1–caster transitions can occur normally in G2-arrested neuroblasts.Citation6
Since cells co-expressing p18 and NICD are surrounded by other cells that undergo normal cell-cycle progression, the change in the former’s temporal character may be affected by the surrounding cells. To what extent is the cell-autonomous timing mechanism in neural progenitor cells influenced by extrinsic cues? We found that neural progenitor cells co-expressing p18 and NICD can be maintained in the single cell state in culture. Moreover, in such cultures, we could observe the actual cell-autonomous timing mechanisms by excluding any effects due to contact with surrounding cells. We found that isolating cells from their neighbors impairs the normal rate of change in both gene expression and laminar fate potential. Interestingly, even in isolated neural progenitors co-expressing NICD and p18, several genes exhibited temporal expression patterns similar to those observed in vivo. These findings lead us to propose a model in which the temporal change in neural progenitor cells is partly mediated by a completely cell-intrinsic mechanism, and that extrinsic cues modify the cell-autonomous change in neural progenitor cells.
Several mechanisms, including a transcriptional regulation cascade,Citation7 epigenetic modification, and subnuclear genome re-organization,Citation2 may be involved in the actual cell-autonomous changes in temporal gene expression. The molecular nature of non–cell-autonomous timing mechanisms is unclear, but some extrinsic factors, such as Fgf10, retinoic acid, and Gde2, are involved in the temporal shift in division mode of neural progenitor cells.Citation2 Further studies are needed to elucidate whether and how these extrinsic cues affect the expression of temporal-axis genes and synchronize the actual cell-autonomous timer in neural progenitor cells.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Toma K, Wang T-C, Hanashima C. Encoding and decoding time in neural development. Dev Growth Differ 2016; 58:59-72; PMID:26748623; http://dx.doi.org/10.1111/dgd.12257
- Kohwi M, Doe CQ. Temporal fate specification and neural progenitor competence during development. Nat Rev Neurosci 2013; 14:823-38; PMID:24400340; http://dx.doi.org/10.1038/nrn3618
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci 2006; 9:743-51; PMID:16680166; http://dx.doi.org/10.1038/nn1694
- Kawaguchi A, Ikawa T, Kasukawa T, Ueda HR, Kurimoto K, Saitou M, Matsuzaki F. Single-cell gene profiling defines differential progenitor subclasses in mammalian neurogenesis. Development 2008; 135:3113-24; PMID:18725516; http://dx.doi.org/10.1242/dev.022616
- Okamoto M, Miyata T, Konno D, Ueda HR, Kasukawa T, Hashimoto M, Matsuzaki F, Kawaguchi A. Cell-cycle-independent transitions in temporal identity of mammalian neural progenitor cells. Nat Commun 2016; 7:11349; PMID: 27094546; http://dx.doi.org/10.1038/ncomms11349
- Grosskortenhaus R, Pearson BJ, Marusich A, Doe CQ. Regulation of temporal identity transitions in Drosophila neuroblasts. Dev Cell 2005; 8:193-202; PMID:15691761; http://dx.doi.org/10.1016/j.devcel.2004.11.019
- Telley L, Govindan S, Prado J, Stevant I, Nef S, Dermitzakis E, Dayer A, Jabaudon D. Sequential transcriptional waves direct the differentiation of newborn neurons in the mouse neocortex. Science 2016; 351:1443-6; PMID: 26940868; http://dx.doi.org/10.1126/science.aad8361
