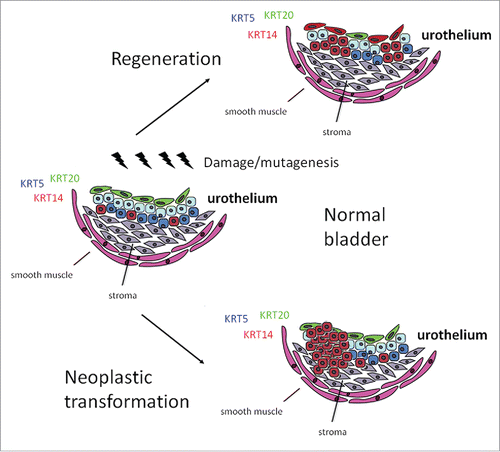
The urothelium is a specialized epithelium with the single role of sealing the luminal side of the urinary tract, forming the urine-blood barrier. It is a slowly cycling epithelium, which however, is able to regenerate within hours upon microbial or chemical injury. The function of the urothelium is executed by its apical or “umbrella” cell layer which is composed of terminally differentiated post-mitotic often bi-nucleated cells lining the inner surface of the urinary tract. Upon natural cycling or injury, these cells shed into the urine, but are replenished immediately. In mice, this process is completed within 72 hours and involves both proliferation and differentiation. Each umbrella cell sits on top of multiple intermediate cells. Basal cells, in direct contact with the lamina propria, are the third urothelial subpopulation.
For many years, it has been postulated that urothelial stem cells able to regenerate all layers lie within the basal layer. Only recently however, with the use of lineage tracing experiments were the investigators able to identify populations with stem cell properties. More specifically, basal cells characterized by Sonic Hedgehog expression (SHH+) give rise to all bladder layers upon chemical injury.Citation1 Basal cells also express Keratin 5 (KRT5). A later report, however, challenged this hypothesis. Gandhi et al reported the existence of 2 distinct subpopulations: a basal SHH+KRT5+ subpopulation responsible for the regeneration of the basal layer, and a SHH+KRT5− intermediate subpopulation that replenishes umbrella cells upon chemical injury.Citation2
In a recent report, we provided conclusive evidence that a subset of exclusively basal KRT5+ cells co-expressing KRT14 are able to regenerate all urothelial layers upon repeated injury with cyclophosphamide (CPP).Citation3 Although at steady state these cells correspond to less than 4% of the total, lineage tracing experiments showed that their descendants are over-represented in all 3 layers upon repeated CPP injury. The KRT5+KRT14+ cells enter the cell cycle first, less than 24 hours from injury. However, after a single challenge with CPP, only rare umbrella cells can be lineage traced back to the KRT5+KRT14+ subpopulation. Following repeated challenges with CPP, 1/5 of umbrella cells are descendants of the initially labeled basal cells. This is of course an underestimation because co-staining with antibodies against KRT14 indicated that only a portion of true KRT14+ cells could be labeled for lineage tracing. Upon challenge, a massive wave of cell proliferation, involving at its peak over 50% of the cells, takes place. While proliferation peaks at 48 hrs post CPP challenge, expression of the umbrella cell marker KRT20 starts at 24 hrs, and at 48 hrs a newly formed umbrella layer is in place. The kinetics implies that the umbrella layer is initially repaired from intermediate cells right underneath. These intermediate cells are themselves replaced later on by basal cells, which explains why after repeated challenges, descendants of KRT5+KRT14+ cells are located in the intermediate and umbrella layer. The ability of KRT14+ cells to support growth was confirmed in vitro with the use of explant cultures as well as clonogenic assays. Finally, an unbiased chemical carcinogenesis model revealed that, although expressed in a small minority, KRT14 marks the cells of origin in at least 50% of the cases.
The observation that a subset of KRT5+ cells co-expressing KRT14 participates in the regeneration of the umbrella layer contradicts reports hypothesizing the existence of distinct progenitors for the basal and umbrella layers in both the mouse and human urothelium.Citation2,4 To clarify this point, we used an inducible KRT5-Cre mouse line to show that indeed KRT5+ cells have umbrella cell renewing capacity. It is quite possible that a subset of non-basal cells retains some proliferative capacity sufficient to regenerate the aging bladder. Upon acute or prolonged injury, however, progenitors within the basal layer are mobilized and replenish all cell types.
Although KRT14-expressing cells have never been identified in the normal human urothelium, KRT14 marks cancer stem cells in human bladder cancer. On the other hand, KRT14 expression in human bladder cancer has been associated with squamous metaplasia. We observed that the frequency of KRT14-expressing cells declines with age. Although one cannot necessarily extrapolate from mice, the existence of KRT14+ cells in younger disease-free human urothelium merits further investigation.
Recent reports implicate important signaling pathways in bladder homeostasis and response to injury, including the Wnt/β-catenin signaling pathway,Citation1,3 the MAPK cascadeCitation5 and retinoic acid signaling.Citation2 It is quite possible that other major signaling pathways, such as the Notch, that are active in normal urotheliumCitation6 play a role in bladder homeostasis. Previously understudied, the urothelium is attracting significant research interest. It has already become clear that the processes governing normal development, regeneration and tumorigenesis are complex and great challenges are awaiting.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 2011; 472:110-4; PMID:21389986; http://dx.doi.org/10.1038/nature09851
- Gandhi D, Molotkov A, Batourina E, Schneider K, Dan H, Reiley M, Laufer E, Metzger D, Liang F, Liao Y, et al. Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev Cell 2013; 26:469-82; PMID:23993789; http://dx.doi.org/10.1016/j.devcel.2013.07.017
- Papafotiou G, Paraskevopoulou V, Vasilaki E, Kanaki Z, Paschalidis N, Klinakis A. KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat Commun 2016; 7:11914; PMID:27320313; http://dx.doi.org/10.1038/ncomms11914
- Castillo-Martin M, Domingo-Domenech J, Karni-Schmidt O, Matos T, Cordon-Cardo C. Molecular pathways of urothelial development and bladder tumorigenesis. Urologic Oncol 2010; 28:401-8; PMID:20610278; http://dx.doi.org/10.1016/j.urolonc.2009.04.019
- Ling S, Chang X, Schultz L, Lee TK, Chaux A, Marchionni L, Netto GJ, Sidransky D, Berman DM. An EGFR-ERK-SOX9 signaling cascade links urothelial development and regeneration to cancer. Cancer Res 2011; 71:3812-21; PMID:21512138; http://dx.doi.org/10.1158/0008-5472.CAN-10-3072
- Rampias T, Vgenopoulou P, Avgeris M, Polyzos A, Stravodimos K, Valavanis C, Scorilas A, Klinakis A. A new tumor suppressor role for the Notch pathway in bladder cancer. Nat Med 2014; 20(10):1199-205; PMID:25194568; http://dx.doi.org/10.1038/nm.3678