Successful chromosome segregation during mitosis is essential for transmission of genetic information to daughter cells. Chromosome segregation is mediated by spatio-temporally coordinated phosphorylation of proteins located on chromosomes and a defect in this segregation result in chromosomal instability and aneuploidy. During mitosis, chromosomal passenger complex (CPC) is a central player that orchestrates various events, such as chromosome alignment, activation of the spindle assembly checkpoint and cytokinesis.Citation1 This complex comprises of Aurora B kinase and 3 other regulatory subunits such as inner centromere protein (INCENP), Survivin and Borealin. Fine-tuned regulation of Aurora B activation and localization ensures the timely and spatially restricted phosphorylation of substrates that are involved in multiple key steps of mitosis. Although maximal activation of Aurora B at the centromere requires autophosphorylation at T232 within its activation loop, the mechanisms underlying the abrupt phosphorylation at this residue at early mitosis has not yet been fully understood.
Our recent study uncovers a novel role of H2AX, a histone H2A variant in autoactivation of Aurora B. H2AX phosphorylation at serine 121 (H2AX-pS121) accumulates and enhances activation of Aurora B at centromeres.Citation2 Depletion of H2AX in HeLa cells, as well as mouse embryonic fibroblasts from H2AX−/− mice shows a severe growth defect due to mitotic abnormalities, such as chromosome misalignment and lagging chromosomes. These mitotic phenotypes are independent of DNA replication errors that occur through impaired H2AX-dependent S phase checkpoint. Depletion of H2AX severely compromises Aurora B activation showing that reduced phosphorylations of Aurora B at T232 and its downstream targets such as centromeric histone CENP-A.
H2AX possesses a unique C-terminal region with various residues harboring covalent modifications. For example, phosphorylation at S139 (γH2AX) is required for recruitment of various proteins to DNA damage sites.Citation3 Interestingly, a mitotic phosphorylation database demonstrates that H2AX at S121 is phosphorylated in vivo and this phosphorylation reaches a maximum at early mitosis. This serine residue is not conserved in canonical H2A and other H2AX variants. The region surrounding this serine residue in H2AX matches well with the Aurora B consensus phosphorylation motif. With the use of phospho-specific antibodies to H2AX-pS121, we successfully demonstrate that H2AX-pS121 predominantly localizes at mitotic centromeres, especially near the kinetochores. Given that this phosphorylation is severely compromised in Aurora B depleted cells, suggesting that Aurora B is a kinase responsible for H2AX-pS121.
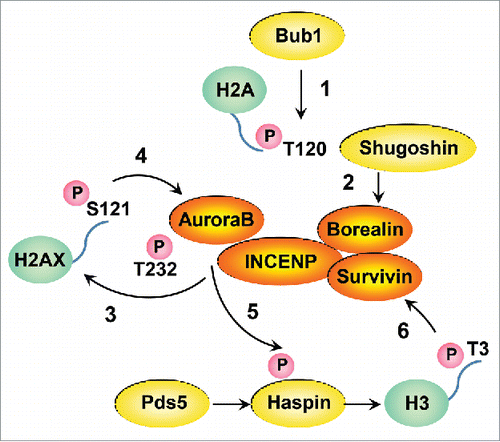
During mitosis, various histones are phosphorylated showing unique spatio-temporal patterns.Citation4 CPC accumulates at the inner centromere during early mitosis through 2 histone phosphorylations such as Haspin-mediated H3-T3 phosphorylation (H3-pT3), and Bub1-mediated H2A-T120 phosphorylation (H2A-pT120).Citation5 Loss of H2AX reduces Aurora B dependent Haspin phosphorylation on chromatin, and subsequently compromises H3-pT3, but does not affect Bub1-mediated H2A-pT120. In contrast, Bub1 is critical for H2AX-pS121. Collectively, these results indicate that Aurora B-mediated H2AX-pS121 facilitates Aurora B autoactivation circuitry at centromeres, acting upstream of Haspin-H3-pT3 and downstream of Bub1-H2A-pT120 (). Supporting this model, a phosphomimic mutant of H2AX at S121 more strongly binds activated Aurora B in vitro when compared with the wild-type counterpart.
Figure 1. Schematical model of Aurora B autoactivation circuitry. Bub1 phosphorylates centromeric H2A at T120 (1). The recruited Shugoshin to H2A-pT120 binds to Borealin (2). Partially activated Aurora B phosphorylates H2AX at S121 (3) leading to its full activation (4), which in turn activates Haspin (5) regulating further deposition of CPC at the inner centromere through Survivin binding(6).
Loss of many mitotic regulators, such as Aurora B and Bub1, leads to embryonic lethality in mice. Paradoxically, despite severe mitotic abnormalities in H2AX or Haspin knockdown cell lines, the knockout mice for H2AX or Haspin are viable. This suggests that, H2AX-pS121 and Haspin-H3-pT3 may act redundantly during the activation of Aurora B at centromeres. Thus, it is of great interest to see whether double-knockout of H2AX and Haspin in mice results in a defect in early embryonic development.
It is interesting to note that several DNA damage responsive proteins, such as ATM and MDC1, are recruited to kinetochore or centromere during mitosis, even in the absence of DNA damage. ATM is phosphorylated and activated by Aurora B in an M phase-dependent manner.Citation6 This phosphorylation is essential for spindle assembly checkpoint via downstream phosphorylation of Bub1. ATM also promotes recruitment of MDC1 at the kinetochores-microtubles attachment during spindle assembly checkpoint by phosphorylating H2AX (γH2AX).Citation7 This in turn induces accumulation of MAD2 and CDC20 at kinetochores. Importantly, aberrant functions of these factors are frequently observed in most cancer cell line. Therefore, it is very exciting to develop the innovative cancer therapies targeting factors regulating centromere and kinetochore integrities.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Carmena M, Wheelock M, Funabiki H, Earnshaw WC. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat Rev Mol Cell Biol 2012; 13:789-803; PMID:23175282; http://dx.doi.org/10.1038/nrm3474
- Shimada M, Goshima T, Matsuo H, Johmura Y, Haruta M, Murata K, Tanaka H, Ikawa M, Nakanishi K, Nakanishi M. Essential role of autoactivation circuitry on Aurora B-mediated H2AX-pS121 in mitosis. Nat Commun 2016; 7:12059; PMID:27389782; http://dx.doi.org/10.1038/ncomms12059
- Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A, Bonner RF, Bonner W, Nussenzweig A. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol 2003; 5:675-9; PMID:12792649; http://dx.doi.org/10.1038/ncb1004
- Wang F, Higgins JMG. Histone modifications and mitosis: countermarks, landmarks, and bookmarks. Trends Cell Biol 2013; 23:175-84; PMID:23246430; http://dx.doi.org/10.1016/j.tcb.2012.11.005
- Watanabe Y. Geometry and force behind kinetochore orientation: lessons from meiosis. Nat Rev Mol Cell Biol 2012; 13:370-82; PMID:22588367; http://dx.doi.org/10.1038/nrm3349
- Yang C, Tang X, Guo X, Niikura Y, Kitagawa K, Cui K, Wong STC, Fu L, Xu B. Aurora-B mediated ATM serine 1403 phosphorylation is required for mitotic ATM activation and the spindle checkpoint. Mol Cell 2011; 44:597-608; PMID:22099307; http://dx.doi.org/10.1016/j.molcel.2011.09.016
- Eliezer Y, Argaman L, Kornowski M, Roniger M, Goldberg M. Interplay between the DNA damage proteins MDC1 and ATM in the regulation of the spindle assembly checkpoint. J Biol Chem 2014; 289:8182-93; PMID:24509855; http://dx.doi.org/10.1074/jbc.M113.532739
