While major research efforts are underway to tackle first world diseases such as cancer, diabetes, and neurodegeneration, parasite-borne diseases preferentially affecting less developed countries are not receiving similar attention, being designated by the World Health Organization as “Neglected.” Human African Trypanosomiasis (HAT), for example, is responsible for 10,000 death/year in sub-Saharan Africa. HAT, also known as sleeping sickness, is characterized by neurological symptoms including sleep disorder and limb paralysis, progressing to coma and eventually death if left untreated. HAT is caused by infection with the protozoan parasite Trypanosoma brucei, unicellular organisms that are transmitted by the tsetse fly. Initially found in the patients' bloodstream, the parasite eventually crosses the blood brain barrier in the later stages of the disease. The drugs currently used for treating HAT are unspecific, and have damaging side effects. Thus, there is an unmet need for identifying new molecular targets to allow the rational design of drugs specifically attacking the parasite.Citation1 In the current volume of Cell Cycle, Mackey and collaborators describe a unique mechanism that regulates proliferation of T. brucei, which may be exploited for therapeutic purposes.Citation2
Since kinases regulate essential cellular processes and they have been extensively studied for chemical inhibition, the authors reasoned that specifically targeting T. brucei kinases may be a good start for developing new therapies. They previously performed a genetic siRNA-based screen targeting 31 uncharacterized kinase genes in T. brucei.Citation3 One of the 2 kinase genes found to be essential for the parasite proliferation was Tb927.10.540, bearing significant homology to the human MAP kinase family member ERK8. Human ERK8 (HsERK8) promotes oncogenic transformation through several mechanisms, including: positive regulation of cell proliferation, suppression of the DNA damage response, activation of autophagy.Citation4,5 HsERK8 was shown to interact with human Proliferating Cell Nuclear Antigen (PCNA),Citation5 an essential regulator of DNA synthesis and DNA repair during S-phase.Citation6 This interaction is essential to prevent PCNA from degradation, and thus promotes DNA synthesis and cell proliferation. Perhaps surprisingly, HsERK8 does not seem to phosphorylate PCNA.
In their current paper,Citation2 Mackey and co-workers investigate the relationship between PCNA and ERK8 in T. brucei. First of all, they find that, similarly to the situation in human cells, TbPCNA and TbERK8 interact directly. This is not surprising, since previous workCitation5 found that HsERK8 has a specific PCNA interaction domain, called a PIP-box,Citation6 which the authors found to be conserved in TbERK8. The interaction was in fact rather weak, which can perhaps be explained by the fact that TbERK8 PIP-box is very degenerate. Next, the authors investigated the catalytic activity of TbERK8. Similar to its human counterpart, TbERK8 showed autophosphorylation activity toward the T174 and Y176 residues in its activation loop consensus sequence T-X-Y. This indicates that TbERK8 can self-activate through autophosphorylation, and further confirms the correct assignment of Tb927.10.540 to the ERK8 family, known to possess this characteristic. Importantly, and rather unexpectedly, TbERK8 also showed activity toward TbPCNA. Indeed, TbPCNA purified from the parasite was found to be phosphorylated at T202 and S216. Moreover, in vitro experiments showed that TbERK8 can phosphorylate TbPCNA at the same residues, and in addition at S211. Crucially, none of these residues were found to be phosphorylated in parasites depleted of TsERK8 by RNA interference, indicating that TbERK8 is indeed the kinase responsible for these modifications.
Interestingly, all 3 residues lay in a unique region of TbPCNA that is predicted to form an extended backside loop, which is not conserved in HsPCNA. In line with this, and further confirming the specificity of this reaction, TbERK8 was not able to phosphorylate HsPCNA. These findings raised the possibility that small molecule inhibitors may differentially affect TbERK8 and HsERK8. In a proof of principle experiment, the authors showed that indeed, a known HsERK8 inhibitor has IC50 values between 100 and 300-fold higher for TbERK8 than HsERK8, arguing that selective inhibitors can be obtained ().
Figure 1. Effects of ERK8 on PCNA. In human cells, ERK8 binds to PCNA and stabilizes it against degradation without phosphorylating it. In T. brucei, ERK8 phosphorylates PCNA to promote cell proliferation through an unknown mechanism. It may also promote PCNA stability. Specific inhibitors for TbERK8 may compromise proliferation of the parasite without side effects caused by inhibition of HsERK8.
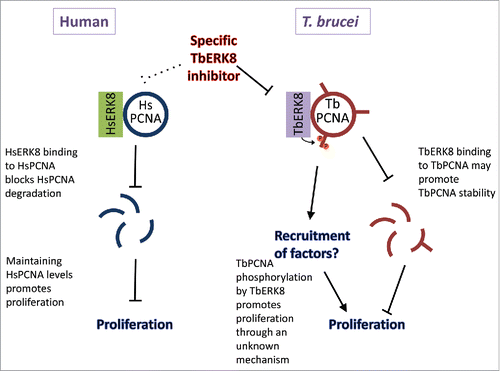
Developing specific TbERK8 inhibitors is an obvious next step. But the results presented here also open other research directions. A particularly important question is what is the function of TbPCNA phosphorylation? It is likely to regulate parasite proliferation, but it will be important to mechanistically understand this PCNA activity. Similar to HsPCNA post-translational modification with mono-ubiquitination or SUMO,Citation7 TbPCNA phosphorylation may recruit specific binding factors. It is also possible that PCNA phosphorylation is important for its stability. Indeed, previously phosphorylation of human PCNA at Y211 by the tyrosine kinase EGFR was shown to prevent degradation of chromatin-bound PCNA.Citation7 In line with this, TbPCNA levels seem to decrease following knockdown of TbERK8.Citation2 However, considering the position of TbPCNA phosphorylation in the unique insertion loop, an effect of ERK8 on PCNA stability independent of its phosphorylation, similar to the situation described for their human counterparts, cannot be ruled out. Regardless of how it is achieved, a reduction in PCNA levels would greatly compromise viability of T. brucei and thus represents a desired outcome in HAT therapy. In conclusion, this paper identified a novel mechanism for regulating proliferation of T. brucei through PCNA phosphorylation by ERK8, which may be specifically targeted for treating trypanosomiasis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Brun R, Blum J, Chappuis F, Burri C. Human African trypanosomiasis. Lancet 2010; 375:148-59; PMID:19833383; http://dx.doi.org/10.1016/S0140-6736(09)60829-1
- Valenciano A, Knudsen GM, Mackey ZB. Extracellular-signal regulated kinase 8 of Trypanosoma brucei uniquely phosphorylates its proliferating cell nuclear antigen homolog and reveals exploitable properties. Cell Cycle 2016; 15(20):2826-40; http://dx.doi.org/10.1080/15384101.2016.1222340
- Mackey ZB, Koupparis K, Nishino M, McKerrow JH. High-Throughput Analysis of an RNAi Library Identifies Novel Kinase Targets in Trypanosoma brucei. Chem Biol Drug Des 2011; 78:454-463; PMID:21668652; http://dx.doi.org/10.1111/j.1747-0285.2011.01156.x
- Rossi M, Colecchia D, Ilardi G, Acunzo M, Nigita G, Sasdelli F, Celetti A, Strambi A, Staibano S, Croce CM, et al. MAPK15 upregulation promotes cell proliferation and prevents DNA damage in male germ cell tumors. Oncotarget 2016; 7:20981-98; PMID:26988910; http://dx.doi.org/10.18632/oncotarget.8044
- Groehler AL, Lannigan DA. A chromatin-bound kinase, ERK8, protects genomic integrity by inhibiting HDM2-mediated degradation of the DNA clamp PCNA. J Cell Biol 2010; 190:575-86; PMID:20733054; http://dx.doi.org/10.1083/jcb.201002124
- Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell 2007; 129:665-79. PMID:17512402; http://dx.doi.org/10.1016/j.cell.2007.05.003
- Wang SC, Nakajima Y, Yu Y-L, Xia W, Chen C-T, McIntush EW, Li L-Y, Hawke DH, Kobayashi R, et al. Tyrosine phosphorylation controls PCNA function through protein stability. Nat Cell Biol 2006; 8:1359-68; PMID:17115032; http://dx.doi.org/10.1038/ncb1501
