ABSTRACT
Tumors are comprised of malignant cancer cells and stromal cells which constitute the tumor microenvironment (TME). Previous studies have shown that cancer associated fibroblast (CAF) in TME is an important promoter of tumor initiation and progression. However, the underlying molecular mechanisms by which CAFs influence the growth of colorectal cancer cells (CRCs) have not been clearly elucidated. In this study, by using a non-contact co-culture system between human colorectal fibroblasts (CCD-18-co) and CRCs (LoVo, SW480, and SW620), we found that fibroblasts existing in tumor microenvironment positively influenced the metabolism of colorectal cancer cells, through its autophagy and oxidative stress pathway which were initially induced by neighboring tumor cells. Therefore, our data provided a novel possibility to develop fibroblasts as a potential target to treat CRC.
Introduction
Colorectal cancer (CRC) was one of the most common malignant disease with characteristics of rapid progression, poor curative effect, and unfavorable prognosis.Citation1,2 According to the statistic results, cure rate of CRC improved slightly compared with its increasing morbidity and mortality.Citation3,4 Therefore, it was necessary to make deep exploration into the high-risk factors leading to cancer formation and find efficient way to get its morbidity and mortality under control.
Since the unlimited proliferation of tumor cells needs to consume too much energy and materials, metabolism of tumor and its microenvironment cells were confirmed to proceed through a special way differ from normal cells. It has been found that tumor cells have increased glycolysis and lactate production compared to normal cells even in the presence of enough oxygen. This phenomenon in which aerobic glycolysis served as a major contributor to total ATP in certain types of cancer cells has been termed the “Warburg Effect”.Citation5,6 In the presence of oxygen, most cells primarily metabolize glucose to pyruvate via glycolysis and then completely oxidize most of this pyruvate to carbon dioxide through the mitochondrial tricarboxylic acid cycle (TCA cycle), where oxygen is the final acceptor in the electron transport chain that generates an electrochemical gradient to facilitate ATP production.
Recently a number of cancer researchers have attempted to understand another aberrant tumor metabolism “The Reverse Warburg Effect,” as the conventional Warburg Effect was thought to be confined to cancer cells and not to occur in stromal fibroblasts. Tumorigenesis was closely related with its microenvironment, which was identified as “cancer cell nests” constituted by malignant cancer cells, immune cells, stromal cells and their secreted cytokines.Citation7-9 Cancer associated fibroblasts (CAFs) of tumor microenvironment were reported to promote tumor initiation, progression and metastasis by interfering cellular metabolism.Citation10-12 CAFs could supply energy-rich metabolites such as lactate through aerobic glycolysis pathway, which can then be used by adjacent cancer cells for ATP generation and for biosynthesis. This results in a unilateral, vectorial, net energy transfer from the catabolic tumor stroma to anabolic cancer cells. Such changes of CAFs might be caused by autophagy when fostered under oxidative stress or tumor induction, in favor of the cancer cells.Citation13-16 CAFs are also able to stimulate cancer cell proliferation and progression through multiple mechanisms by secretion of variety of cytokines, chemokines and extracellular matrix components. These, in turn, stimulate both receptor tyrosine kinase signaling and the synthesis of proteins involved in the extracellular matrix remodeling. Orimo et al. found that CAFs could promote breast cancer cell growth and angiogenesis by secreting stromal cell-derived factor 1 and recruiting endothelial progenitor cells.Citation17 Furthermore, as a source of extracellular matrix-degrading proteases, CAFs promote disaggregation of normal mammary epithelial cells and trigger epithelial-mesenchyme transition-rendered tumorigenesis and invasiveness.Citation18 The presence of CAFs in oral squamous cell carcinoma (OSCC) was reported to be associated with OSCC invasiveness and prognosis.Citation19 Despite the fact that a number of studies have confirmed the role of CAFs in supporting tumor growth and progression in various types of carcinomas, including oral, breast, prostate and lung, the effect of CAFs on CRCs tumor progression and on the chemokines or cytokines signal pathways involving in CAFs-colorectal cancer cells (CRCs) cross-talk remains largely uncharacterized.Citation20-22
Cancer cells undergo a metabolic reprogramming which might explain why it could not lead to long-term efficient therapeutic effects when using chemotherapeutic drugs targeting the “seeds”-cancer cells, or using antiangiogenic drugs such as vascular endothelial growth factor (VEGF) inhibitor.Citation23,24 However, little is known about metabolic alterations of stromal cells within tumors. CAFs are pro-tumorigenic although the mechanisms by which CAFs support tumors have not been elucidated.
The aim of this study was to investigate the regulatory mechanism underlying the interaction between CRCs and CAFs. A non-contact co-culture system between fibroblasts and CRCs in vitro was established to mimic the tumor microenvironment, which might influence energy metabolism during colorectal tumorigenesis. By exploring the underlying mechanisms of CAFs metabolic reprogramming, we could make a better understanding of tumor growth environment and provide new evidence for future treatment of colorectal cancer.
Materials and methods
Cell preparations
Human CRC cell lines LoVo (ATCC, CCL-229), SW480 (ATCC, CCL-228), SW620 (ATCC, CCL-227) and Human colorectal fibroblast CCD-18-co (ATCC,CRL-1459) were authenticated in April 2014 and cultured in Dulbecoo minimum essential medium (DMEM) high-glucose medium (HyClone, SH30243.01B) supplemented with 10% fetal bovine serum (Gibco, Grand Island, New York, USA) and 1% penicillin-streptomycin (Beijing DingGuoChangSheng Biotechnology, CC-0113).
Co-culture of CRCs and human colorectal fibroblasts
0.4 μm transwell apparatus(Corning, 00915038)were used to co-culture cancer cells and fibroblasts without direct contact in serum free medium. Fibroblasts and cancer cells were initially seeded into upper inserts (2.5 × 104/insert) and lower 6-well plate (1.0 × 105/well) as required respectively, which were fit together when the cells become adherent overnight. After co-cultured for 48h, cells in the 6-well plate were harvested for subsequent cell counting and protein extracting. Cells treated with DMEM medium only were considered as controls.
Cell viability assay
The cell viability of colorectal cancer cells and fibroblasts was measured by MTT assay. CRCs (LoVo, SW480, and SW620) and CCD-18-co cell were cultured respectively and maintained in 5% CO2 at 37°C until reaching 60–80% confluence, then the culture medium was discarded and fresh medium was added. Cells were harvested and all culture medium were collected 24 h later respectively. Colorectal cancer cells were subsequently suspended with gradient concentration of CCD-18-co-medium diluted by fresh DMEM medium (0, 10%, 20%, 40%, 50%, 60%, 80%, 100%). Meanwhile, CCD-18-co cells were suspended with gradient concentration of colorectal cancer cell culture medium (LoVo, SW480, and SW620) diluted by fresh DMEM medium (0, 10%, 20%, 40%, 50%, 60%, 80%, 100%). Cells were seeded into 96-well plate 2000/well for 48 h. Ten ul of MTT (5 mg/ml in PBS; all Sigma-Aldrich) solution was added to each well and incubated at 37°C for 4 h. Absorbance of the solution was measured at 570 nm (indicating cell viability) using a 96-well plate reader as previously described.Citation25 The cell viability was calculated as follows: Cell Survival Rate (CSR) (%) = Treatment group A570/Control group A570 × 100%All assays were done with 6 parallel samples.
Western blot analysis
Total proteins from the cells were extracted with RIPA lysis buffer. The cells were collected by centrifugation at 500 rpm for 5 min and then homogenized with 0.9 mL of normal saline using an electric homogenate machine. Protein concentrations were measured by BCA Assay Kit (Beyotime, P0010) in accordance with the specifications. The proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. After blocking, the membrane were incubated with specific primary antibodies against IDH2 (Santa Cruz, SC-134923), PKM2 (Bioworld Technology, BS6443), GAPDH (Santa Cruz, SC-365062), GSTP1 (Santa Cruz, SC-376481), SOD2 (Santa Cruz, SC-130345), BNIP3 (Abcam, ab10433), and LC3 (Novusbio, NB100-2220), followed by anti-mouse or rabbit horseradish peroxidase-conjugated IgG (ZSGB-BIO, ZB-2301/ZB-2305) and developed with the chemiluminescence method (ECL, Millipore). β-actin (Beijing Biosynthesis Biotechnology, BS-0061R) served as the loading control.
Assay of enzyme activity
Total proteins were harvested to assay the enzyme activities. The entire process of grinding was performed on ice to preserve the enzymatic activity. The activities of PKM2 and IDH2 were measured with Assay Kits for the detection of PKM2 (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and IDH2 (MAK062-1KT, Sigma) according to the manufacturer's instructions.
Confocal immunofluorescence microscopy
The confocal measurement was conducted to observe the expression of the target proteins in cells under different conditions: Colorectal cancer cells and fibroblasts were co-cultivated in a 24-wells transwell apparatus (Corning, 35314024) with or without the oxidative stress inhibitor NAC 8 mM (Beyotime, S0077) and autophagy inhibitor 3-MA 5 mM (sigma, M9281). After co-cultured for 48 h, slides with adhered cells in the 24-well plates were collected and fixed in 1:1 methanol and acetone mixture, blocked with 3% BSA for 20 min at room temperature, and permeabilized with 10% Triton-X-100. Cells were stained with primary antibodies against SOD2, LC3, PKM2, IDH2 and GAPDH, which were subsequent detected with Alexa Fluor-conjugated secondary antibodies. Cell nucleus were shown using DAPI staining (Life Technologies). Stained cells were viewed using Zeiss LSM 700 fluorescence confocal microscope. Images were analyzed using the ZEN digital imaging software (Zeiss, German).
Statistical analysis
The data were presented as the mean ± SDM. The samples were analyzed using a 2-tailed unpaired Student's t-test, unless otherwise noted, and statistical analyses were performed with the Statistical Package for the Social Sciences, version 17.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Cell proliferation was promoted in co-culture system
To explore the influence of stroma microenvironment on colorectal carcinogenesis, a non-contact co-culture system was adopted to mimic the interactions between CAFs and CRCs. The results showed that the number of both colorectal cells and fibroblasts increased () by cell counting in the non-contact co-culture system ().
Figure 1. The growth of cells in co-culture system was measured by cell counting chamber and MTT assay. (A) Observation of colorectal cancer cells LoVo, SW480, SW620 cultured alone (left) or co-cultured with CCD-18-co (right) under an optical microscope (40×). (C) Observation of CCD-18-co cultured alone or co-cultured with cancer cells under an optical microscope (200×). (B&D) Cells were harvested and counted by cell counting chamber. **P< 0.01 versus negative.
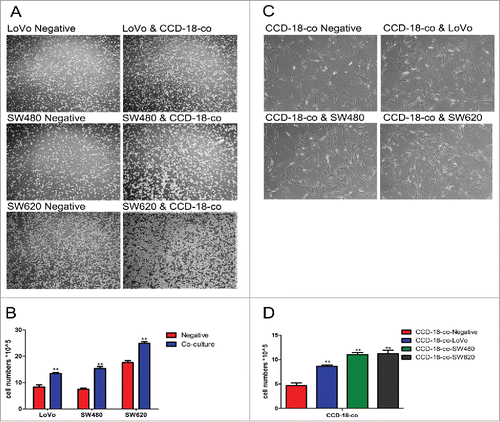
MTT assay revealed that the viability of CRCs increased with the increased concentration of CCD-18-co culture medium (). showed that the viability of CCD-18-co cells could be promoted along with the increased concentration of CRCs culture medium. Our results suggested that there was a mutually reinforcing relationship between colorectal cancer cells and stroma microenvironment fibroblasts.
Table 1. Effects of CCD-18-co-medium on cell viability of colorectal cancer cells (LoVo, SW480, and SW620).
Table 2. Effects of colorectal cancer cells (LoVo, SW480, and SW620) medium on cell viability of CCD-18-co.
Metabolic changes in both co-cultured fibroblasts and colorectal cancer cells
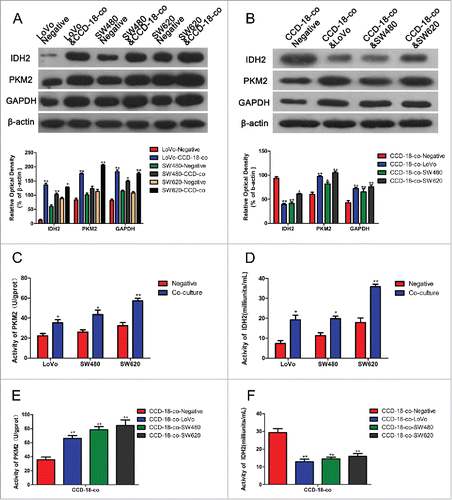
Cell proliferation could be promoted by metabolic changes. In order to investigate the effect of metabolic changes on cell proliferation, the protein expression of metabolic enzymes were measured. The results showed that expression of both enzymes involved in KREBS cycle (IDH2) and glycolytic pathway (PKM2 and GAPDH) were up-regulated in co-cultivated CRCs. However, the expression of IDH2 was significantly decreased in co-cultivated fibroblasts with the same increased PKM2 and GAPDH expression (). The measurement of metabolic enzymes' activities by using PKM2 and IDH2 activity assay kits obtained the same results ().
Figure 2. Expression changes of metabolism related proteins were measured by Western blot and enzyme activity assay kits. (A) The expression of proteins involved in KREBS cycle and glycolytic pathway in colorectal cancer cells. The two neighboring columns represent expression of indicated proteins in cancer cell lines co-cultured with CCD-18-co compared to their control groups, respectively. β-actin served as control for normalization. *P< 0.05 vs. corresponding negative respectively, **P < 0.01 versus corresponding negative respectively. (B) The expression of proteins involved in KREBS cycle and glycolytic pathway in CCD-18-co cultured with cancer cells or not. β-actin served as control for normalization. *P < 0.05 vs. negative respectively, **P < 0.01 versus negative respectively. (2C&2D) Analysis of PKM2 and IDH2 activities in 3 tumor cell lines. *P < 0.05 vs. negative respectively, **P < 0.01 versus negative respectively. (2E&2F) Analysis of PKM2 and IDH2 in CCD-18-co cultured alone or co-cultured with tumor cells. *P < 0.01 vs. negative respectively.
Expression of the oxidative stress key enzymes and autophagy related proteins in co-cultured CCD-18-co
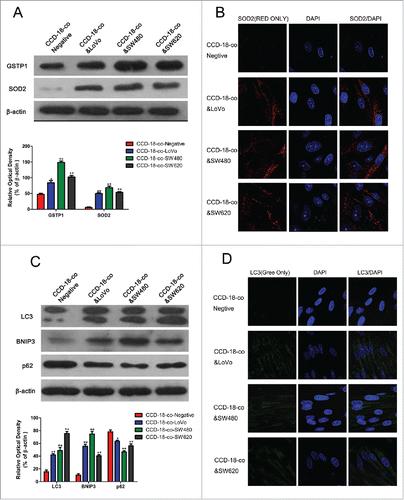
To explore the underlying mechanism of upregulated glycolysis but a reversed activity of KREBS cycle in colorectal cancer cells and fibroblasts, expression of oxidative stress and autophagy related proteins in fibroblasts co-cultured with colorectal cancer cells were investigated by western blot and immunofluorescence. As shown in , expression of oxidative stress related proteins SOD2 and GSTP1 increased in CCD-18-co cells co-cultured with 3 kinds of colorectal cancer cells. Expression of autophagy related proteins LC3, BNIP3, and p62 also increased in CCD-18-co cells co-cultured with these cancer cells (). And we have shown that fibroblasts co-cultured with cancer cells could accumulate vesicular structures that we have identified as autophagosomes by immuno-staining with the autophagy marker LC3 ().
Figure 3. Expression of oxidative stress and autophagy related proteins in fibroblasts co-cultured with colorectal cancer cells were measured by western blot and immunofluorescence. (A) Expression of oxidative stress related proteins SOD2 and GSTP1 in CCD-18-co cells co-cultured with tumor cells, β-actin served as loading control. *P < 0.05 versus negative respectively, **P < 0.01 vs. negative respectively. (B) Immunofluorescence observation of SOD2 (red) in CCD-18-co cells co-cultured with tumor cells (63×). (C) Expression of autophagy related proteins LC3, BNIP3 and p62 in CCDs co-cultured with tumor cells. **P < 0.01 versus negative respectively. (D) Immunofluorescence observation of LC3 (green) in CCDs co-cultured with tumor cells (63×).
Detection of protein expression in the presence of oxidative stress inhibitors NAC or autophagy inhibitor 3-MA
To determine whether the metabolic changes were associated with oxidative stress and autophagy occurred in tumor microenvironment, the oxidative stress inhibitor NAC (8mM) or autophagy inhibitor 3-MA (5mM) was used to treat fibroblast CCD-18-co when co-cultured with tumor cells. showed that the expression of SOD2 or LC3 could be inhibited by NAC or 3-MA in colorectal cancer cells co-culture with CCD-18-co. Meanwhile, the abnormal expression of metabolism related proteins in CCD-18-co had been reversed by oxidative stress inhibitor and autophagy inhibitor ().
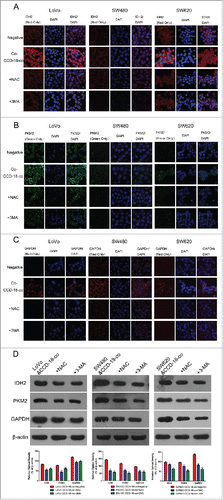
Figure 4. Expression changes of metabolic related proteins in CCD-18-co after being treated with inhibitor NAC or 3-MA. (A) Immunofluorescence observation of SOD2 (red) in co-cultured CCD-18-co cells in the presence of oxidative stress inhibitor NAC or autophagy inhibitor 3-MA (63×). (B) Immunofluorescence observation of LC3 (green) in co-cultured CCD-18-co cells in the presence of oxidative stress inhibitors NAC or autophagy inhibitor 3-MA (63×). (C) Expression of metabolism related proteins (IDH2, PKM2, and GAPDH), oxidative stress related proteins (SOD2 and GSTP1) and autophagy related proteins (LC3, BNIP3, and p62) in CCDs co-cultured with colorectal cancer cells under treatment of NAC or 3-MA, respectively. β-actin served as loading control. *P < 0.05 vs. negative respectively, **P < 0.01 versus negative respectively.
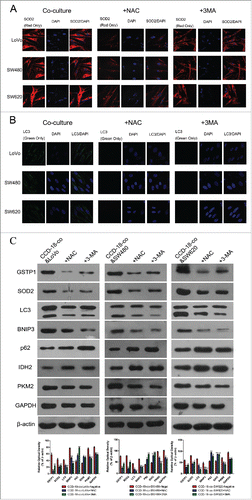
Similarly, oxidative stress inhibitor NAC (8mM) or autophagy inhibitor 3-MA (5mM) was used to treat colorectal cancer cells when co-cultured with fibroblast CCD-18-co. The abnormal expression of metabolism related proteins in colorectal cancer cells had been reversed by oxidative stress inhibitor and autophagy inhibitor ().
Figure 5. Expression changes of metabolic related proteins in tumor cells after being treated with inhibitor NAC or 3-MA by using western blot and immunofluorescence. (D) Expression of metabolism related proteins IDH2, PKM2, GAPDH in colorectal cancer cells co-cultured with CCD-18-co under treatment of NAC or 3-MA, respectively. *P < 0.05 vs. negative respectively, **P < 0.01 versus negative respectively. (A, B, and C) Immunofluorescence observation of metabolism related proteins GAPDH, PKM2, IDH2 in tumor cells cultured alone or co-cultured with CCD-18-co under treatment of NAC or 3-MA, respectively (63×).
Discussion
The tumor microenvironment that is composed of several cell-types, including fibroblasts, immune cells, endothelial cells and adipocytes has recently gained much attention as a critical determinant of tumor progression and clinical outcome.Citation26 Cancer initiation and progression are largely dependent on the physical and chemical features of the adjacent environment. CAFs have been described to play critical roles in initiation, progression and metastasis of various cancers. Olumi et al. found that CAFs could induce formation of tumors when simian virus 40-transformed prostate epithelial cells BPH-1 were coinjected with either CAFs or normal fibroblasts, indicating that CAFs can initiate tumorigenesis.Citation27-29 Similarly, neighboring CAFs could promote proliferation, migration and growth of oral cancer cells (OCCs) through enhancing the expression of cell cycle-driven proteins.Citation30 However, the involvement of CAFs in CRCs has not been well addressed. Our study showed that proliferation of colorectal cancer cells co-cultured with fibroblasts or treated with fibroblasts-conditioned medium increased, meanwhile a paralleled promotion was also observed in fibroblasts co-cultured with colorectal cancer cells, which means there are synergistic interactions between tumor cells and microenvironmental fibroblasts.
It is becoming increasingly clear that uncontrolled proliferation of cancer cells is generally associated with altered energy metabolism. Tumor cells have special metabolic requirements for their development into 3-dimensional tumor masses. Glucose is a primary source of energy, which can be converted to pyruvate via glycolysis in the cytosol, and subsequently oxidized to produce ATP in the mitochondria under aerobic conditions. Under anaerobic conditions, conversion of pyruvate to lactic acid is favored with relatively low amounts of pyruvate being diverted to the mitochondria. However, cancer cells primarily acquire energy from glucose via glycolysis to produce lactic acid, even under aerobic conditions, a property first observed by Otto Warburg.Citation31 This “aerobic glycolysis,” also known as the “Warburg effect”,Citation32 is much less energy-efficient than the oxidative phosphorylation pathway.Citation33 A recent study showed that cancer cells may shift metabolic pathways to facilitate the uptake and incorporation of nutrients into cell building blocks, such as nucleotides, amino acids and lipids, that are needed for highly proliferating cells.Citation34 In this study, we estimated expression of metabolism related enzymes in CRCs and fibroblasts respectively in a co-culture system. We found that expression of GAPDH, PKM2, and IDH2 in CRCs were all unregulated but IDH2 in fibroblasts was downregulated diversely. All these results indicated that CRCs in co-culture system appeared a stimulated metabolism phenotype of both glycolytic and oxidative phosphorylation pathway, while fibroblasts in co-culture system presented a restricted oxidative phosphorylation pathway and an enhanced glycolytic pathway which would serve as the main energy source of cell proliferation. All these characters were quite another thing from the traditional “Warburg effect.” CAFs might undergo alterations in carbohydrate metabolism to support the proliferation of cancer cells. Through glycolysis pathway, CAFs could generate a lot of high-energy intermediate metabolites (pyruvic acid/lactic acid. etc.), which served as raw materials to synthesize proteins and lipids required in the process of tumor growth and proliferation. Similar results in lung cancer associated fibroblasts have also been confirmed by Virendra K. Chaudhri et al.Citation35 Our study revealed that tumor stromal fibroblasts play an important role in metabolic reprogramming of CRCs.
A recent study found that breast cancer cells could induce oxidative stress in adjacent cancer associated fibroblasts, resulting in the autophagy/lysosomal degradation of stromal Caveolin-1 (Cav-1) in a co-culture model. A loss of Cav-1 triggers HIF-1α stabilization in CAFs, with increased aerobic glycolysis and enhanced autophagy.Citation36 Similarly in our study, we demonstrated an up-regulated expression of oxidative stress related enzymes in fibroblasts co-cultured with colorectal cancer cells by western blotting and immunofluorescence. Meanwhile, an increased expression of autophagy related proteins (LC3 and BNIP3) and a reduced autophagy metabolites and Cav-1 were also presented in fibroblasts (Cav-1 in Fig. S1A). Although lots of studies focused on the regulation of cancer cell metabolism, whether or not does oxidative stress or autophagy play a key role in metabolic changes of both colorectal cancer cells and fibroblasts were unclear. When oxidative stress inhibitors were added to the medium, we found reduced autophagy and proliferation, down-regulated glycolysis enzymes, and restoring expression of IDH2 and Cav-1 (Cav-1 in Fig. S1B) in co-cultured CAFs. However in co-cultured CRCs, both glycolysis enzymes and IDH2 expression were all downregulated to the levels of CRC cultivated alone in the presence of oxidative stress inhibitors. Numerous studies have suggested that inhibition of reactive oxygen species (ROS) production in cancer cells might be an effective therapeutic strategy for cancer therapy.Citation37-39 A new study suggests that overexpression of catalase in CAFs-OCCs co-culture abolished production of ROS, providing a novel evidence for “anti-oxidant therapy”.Citation30 When autophagy inhibitors were added to the medium, we detected the similar results. And rapamycin, a clinically approved drug, inhibit cancer by targeting stromal fibroblasts.Citation40,41 Meanwhile a study found that NAC inhibits mTOR.Citation42 And the results in our study demonstrated that oxidative stress induced by colorectal cancer cells could stimulate autophagy in CAFs, which then led to intensive glycolysis and attenuated aerobic oxidation. The high-energy compounds (pyruvic acid/lactic acid) generated in glycolysis were either supplied to fast-growing tumor cells or used to maintain the upregulated autophagy state of CAFs themselves.Citation43,44
In summary, this study provided evidences that colorectal cancer cells could induce oxidative stress in microenvironmental fibroblasts, which were then undergone metabolic changes, including increased expression of glycolytic enzymes, reduced KREBS cycle enzymes and autophagy. Oxidative stress inhibitors and autophagy inhibitors could inhibit the metabolic reprogramming between tumor cells and fibroblasts. Increased autophagy is believed to promote survival of cancer cells by providing nutrients (pyruvic acid/lactic acid) for cancer proliferation and/or as a protection against oxidative damage. Studies of autophagy in tumors have focused on cancer cells.Citation38 However our studies provided evidence that an autophagy was increased in fibroblasts of the tumor microenvironment, suggesting that alterations of autophagy within tumors were not restricted to the cancer cells. Increased CAFs autophagy might not only provide a survival benefit for CAFs in the tumor, but also contribute to the protumorigenic effects of CAFs, since CAFs thriving in the tumor would be able to support cancer cells and tumor growth. Therefore, oxidative stress and autophagy inhibitors might be used as potential drugs for the treatment of colorectal cancer, by interfering with metabolic reprogramming of the tumor microenvironment.
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest.
KCCY_A_1252882_Supplementary_material.zip
Download Zip (372.7 KB)Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2014HM087). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Khan K, Wale A, Brown G, Chau I. Colorectal cancer with liver metastases: neoadjuvant chemotherapy, surgical resection first or palliation alone? World J Gastroenterol 2014; 20:12391-406; PMID:25253940; http://dx.doi.org/10.3748/wjg.v20.i35.12391
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014; 64:104-17; PMID:24639052; http://dx.doi.org/10.3322/caac.21220
- Jia Y, Xu G, Zhou W, Wang Z, Meng L, Zhou S, Xu X, Yuan H, Tian K. Diabetes promotes DMH-induced colorectal cancer by increasing the activity of glycolytic enzymes in rats. PLoS One 2014; 9:e110455; PMID:25329503; http://dx.doi.org/10.1371/journal.pone.0110455
- Li T, Leong MH, Harms B, Kennedy G, Chen L. MicroRNA-21 as a potential colon and rectal cancer biomarker. World J Gastroenterol 2013; 19:5615-21; PMID:24039353; http://dx.doi.org/10.3748/wjg.v19.i34.5615
- Bensinger SJ, Christofk HR. New aspects of the Warburg effect in cancer cell biology. Semin Cell Dev Biol 2012; 23:352-61; PMID:22406683; http://dx.doi.org/10.1016/j.semcdb.2012.02.003
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324:1029-33; PMID:19460998; http://dx.doi.org/10.1126/science.1160809
- Ko H, Son S, Jeon J, Thambi T, Kwon S, Chae YS, Kang YM, Park JH. Tumor microenvironment-specific nanoparticles activatable by stepwise transformation. J Control Release 2016; 234:68-78; PMID:27164544; http://dx.doi.org/10.1016/j.jconrel.2016.05.009
- Sung SY, Chung LW. Prostate tumor-stroma interaction: molecular mechanisms and opportunities for therapeutic targeting. Differentiation 2002; 70:506-21; PMID:12492493; http://dx.doi.org/10.1046/j.1432-0436.2002.700905.x
- Xue T, Liu P, Zhou Y, Liu K, Yang L, Moritz RL, Yan W, Xu LX. Interleukin-6 induced “Acute” phenotypic microenvironment promotes Th1 anti-tumor immunity in Cryo-thermal therapy revealed by shotgun and parallel reaction monitoring proteomics. Theranostics 2016; 6:773-94; PMID:27162549; http://dx.doi.org/10.7150/thno.14394
- Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes Dev 2016; 30:1002-19; PMID:27151975; http://dx.doi.org/10.1101/gad.279737.116
- Shaker H, Bundred NJ, Albadry H, Nicholson S, Castle J, Lumsden LJ, Pritchard S, Landberg G, Kirwan CC. PO-21 - Stromal fibroblasts in preinvasive breast cancer (ductal carcinoma in situ, DCIS) demonstrate a cancer-like procoagulant phenotypic switch that may facilitate invasion. Thromb Res 2016; 140 Suppl 1:S184; PMID:27161710; http://dx.doi.org/10.1016/S0049-3848(16)30154-2
- Verghese ET, Drury R, Green CA, Holliday DL, Lu X, Nash C, Speirs V, Thorne JL, Thygesen HH, Zougman A, et al. MiR-26b is down-regulated in carcinoma-associated fibroblasts from ER-positive breast cancers leading to enhanced cell migration and invasion. J Pathol 2013; 231:388-99; PMID:23939832; http://dx.doi.org/10.1002/path.4248
- Lisanti MP, Martinez-Outschoorn UE, Sotgia F. Oncogenes induce the cancer-associated fibroblast phenotype: metabolic symbiosis and “fibroblast addiction” are new therapeutic targets for drug discovery. Cell Cycle 2013; 12:2723-32; PMID:23860382; http://dx.doi.org/10.4161/cc.25695
- Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, Daumer KM, Milliman JN, Chiavarina B, Migneco G, Witkiewicz AK, Martinez-Cantarin MP, Flomenberg N, et al. Tumor cells induce the cancer associated fibroblast phenotype via caveolin-1 degradation: implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle 2010; 9:2423-33; PMID:20562526; http://dx.doi.org/10.4161/cc.9.12.12048
- Martinez-Outschoorn UE, Trimmer C, Lin Z, Whitaker-Menezes D, Chiavarina B, Zhou J, Wang C, Pavlides S, Martinez-Cantarin MP, Capozza F, et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: Role of hypoxia, HIF1 induction and NFkappaB activation in the tumor stromal microenvironment. Cell Cycle 2010; 9:3515-33; PMID:20855962; http://dx.doi.org/10.4161/cc.9.17.12928
- Sanchez-Alvarez R, Martinez-Outschoorn UE, Lin Z, Lamb R, Hulit J, Howell A, Sotgia F, Rubin E, Lisanti MP. Ethanol exposure induces the cancer-associated fibroblast phenotype and lethal tumor metabolism: implications for breast cancer prevention. Cell Cycle 2013; 12:289-301; PMID:23257780; http://dx.doi.org/10.4161/cc.23109
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005; 121:335-48; PMID:15882617; http://dx.doi.org/10.1016/j.cell.2005.02.034
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 1999; 98:137-46; PMID:10428026; http://dx.doi.org/10.1016/S0092-8674(00)81009-0
- Fujii N, Shomori K, Shiomi T, Nakabayashi M, Takeda C, Ryoke K, Ito H. Cancer-associated fibroblasts and CD163-positive macrophages in oral squamous cell carcinoma: their clinicopathological and prognostic significance. J Oral Pathol Med 2012; 41:444-51; PMID:22296275; http://dx.doi.org/10.1111/j.1600-0714.2012.01127.x
- Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A 2002; 99:12877-82; PMID:12297622; http://dx.doi.org/10.1073/pnas.162488599
- Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, Sudilovsky D, Cunha GR. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res 2001; 61:8135-42; PMID:11719442
- Pena C, Cespedes MV, Lindh MB, Kiflemariam S, Mezheyeuski A, Edqvist PH, Hägglöf C, Birgisson H, Bojmar L, Jirström K, et al. STC1 expression by cancer-associated fibroblasts drives metastasis of colorectal cancer. Cancer Res 2013; 73:1287-97; PMID:23243022; http://dx.doi.org/10.1158/0008-5472.CAN-12-1875
- Guido C, Whitaker-Menezes D, Capparelli C, Balliet R, Lin Z, Pestell RG, Howell A, Aquila S, And∫ S, Martinez-Outschoorn U, et al. Metabolic reprogramming of cancer-associated fibroblasts by TGF-beta drives tumor growth: connecting TGF-beta signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle 2012; 11:3019-35; PMID:22874531; http://dx.doi.org/10.4161/cc.21384
- Sotgia F, Martinez-Outschoorn UE, Pavlides S, Howell A, Pestell RG, Lisanti MP. Understanding the Warburg effect and the prognostic value of stromal caveolin-1 as a marker of a lethal tumor microenvironment. Breast Cancer Res 2011; 13:213; PMID:21867571; http://dx.doi.org/10.1186/bcr2892
- Liu L, Zhang LI, Lin YE, Bian Y, Gao X, Qu BO, Li Q. 14-3-3gamma regulates cell viability and milk fat synthesis in lipopolysaccharide-induced dairy cow mammary epithelial cells. Exp Ther Med 2016; 11:1279-87; PMID:27073437
- Martinez-Outschoorn U, Sotgia F, Lisanti MP. Tumor microenvironment and metabolic synergy in breast cancers: critical importance of mitochondrial fuels and function. Semin Oncol 2014; 41:195-216; PMID:24787293; http://dx.doi.org/10.1053/j.seminoncol.2014.03.002
- Jia CC, Wang TT, Liu W, Fu BS, Hua X, Wang GY, Li TJ, Li X, Wu XY, Tai Y, et al. Cancer-associated fibroblasts from hepatocellular carcinoma promote malignant cell proliferation by HGF secretion. PLoS One 2013; 8:e63243; PMID:23667593; http://dx.doi.org/10.1371/journal.pone.0063243
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 1999; 59:5002-11; PMID:10519415
- Xing F, Saidou J, Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci (Landmark Ed) 2010; 15:166-79; PMID:20036813; http://dx.doi.org/10.2741/3613
- Li X, Xu Q, Wu Y, Li J, Tang D, Han L, Fan Q. A CCL2/ROS autoregulation loop is critical for cancer-associated fibroblasts-enhanced tumor growth of oral squamous cell carcinoma. Carcinogenesis 2014; 35:1362-70; PMID:24531940; http://dx.doi.org/10.1093/carcin/bgu046
- Warburg O. On respiratory impairment in cancer cells. Science 1956; 124:269-70; PMID:13351639
- Feron O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol 2009; 92:329-33; PMID:19604589; http://dx.doi.org/10.1016/j.radonc.2009.06.025
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell 2008; 134:703-7; PMID:18775299; http://dx.doi.org/10.1016/j.cell.2008.08.021
- Casey TM, Eneman J, Crocker A, White J, Tessitore J, Stanley M, Harlow S, Bunn JY, Weaver D, Muss H, et al. Cancer associated fibroblasts stimulated by transforming growth factor beta1 (TGF-beta 1) increase invasion rate of tumor cells: a population study. Breast Cancer Res Treat 2008; 110:39-49; PMID:17674196; http://dx.doi.org/10.1007/s10549-007-9684-7
- Chaudhri VK, Salzler GG, Dick SA, Buckman MS, Sordella R, Karoly ED, Mohney R, Stiles BM, Elemento O, Altorki NK, et al. Metabolic alterations in lung cancer-associated fibroblasts correlated with increased glycolytic metabolism of the tumor. Mol Cancer Res 2013; 11:579-92; PMID:23475953; http://dx.doi.org/10.1158/1541-7786.MCR-12-0437-T
- Shi Y, Tan SH, Ng S, Zhou J, Yang ND, Koo GB, McMahon KA, Parton RG, Hill MM, Del Pozo MA, et al. Critical role of CAV1/caveolin-1 in cell stress responses in human breast cancer cells via modulation of lysosomal function and autophagy. Autophagy 2015; 11:769-84; PMID:25945613; http://dx.doi.org/10.1080/15548627.2015.1034411
- Lampiasi N, Azzolina A, Umezawa K, Montalto G, McCubrey JA, Cervello M. The novel NF-kappaB inhibitor DHMEQ synergizes with celecoxib to exert antitumor effects on human liver cancer cells by a ROS-dependent mechanism. Cancer Lett 2012; 322:35-44; PMID:22343223; http://dx.doi.org/10.1016/j.canlet.2012.02.008
- Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, Whitaker-Menezes D, Daumer KM, Lin Z, Witkiewicz AK, et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 2010; 9:3256-76; PMID:20814239
- Montero AJ, Jassem J. Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs 2011; 71:1385-96; PMID:21812504; http://dx.doi.org/10.2165/11592590-000000000-00000
- Kabir TD, Leigh RJ, Tasena H, Mellone M, Coletta RD, Parkinson EK, Prime SS, Thomas GJ, Paterson IC, Zhou D, et al. A miR-335/COX-2/PTEN axis regulates the secretory phenotype of senescent cancer-associated fibroblasts. Aging (Albany NY) 2016; 8:1608-35; PMID:27385366; http://dx.doi.org/10.18632/aging.100987
- Mercier I, Camacho J, Titchen K, Gonzales DM, Quann K, Bryant KG, Molchansky A, Milliman JN, Whitaker-Menezes D, Sotgia F, et al. Caveolin-1 and accelerated host aging in the breast tumor microenvironment: chemoprevention with rapamycin, an mTOR inhibitor and anti-aging drug. Am J Pathol 2012; 181:278-93; PMID:22698676; http://dx.doi.org/10.1016/j.ajpath.2012.03.017
- Leontieva OV, Blagosklonny MV. Yeast-like chronological senescence in mammalian cells: phenomenon, mechanism and pharmacological suppression. Aging (Albany NY) 2011; 3:1078-91; PMID:22156391; http://dx.doi.org/10.18632/aging.100402
- Lisanti MP, Martinez-Outschoorn UE, Sotgia F. Oncogenes induce the cancer-associated fibroblast phenotype: metabolic symbiosis and “fibroblast addiction” are new therapeutic targets for drug discovery. Cell Cycle 2013; 12:2723-32; PMID:23860382; http://dx.doi.org/10.4161/cc.25695
- Peiris-Pages M, Smith DL, Gyorffy B, Sotgia F, Lisanti MP. Proteomic identification of prognostic tumour biomarkers, using chemotherapy-induced cancer-associated fibroblasts. Aging (Albany NY) 2015; 7:816-38; PMID:26539730
