ABSTRACT
Polyploids are pervasive in plants and have large impacts on crop breeding, but natural polyploids are rare in animals. Mouse diploid embryos can be induced to become tetraploid by blastomere fusion at the 2-cell stage and tetraploid embryos can develop to the blastocyst stage in vitro. However, there is little information regarding mouse octaploid embryonic development and precise mechanisms contributing to octaploid embryonic developmental limitations are unknown. To investigate the genetic and epigenetic mechanisms underlying octaploid embryonic development, we generated mouse octaploid embryos and evaluated the in vitro/in vivo developmental potential. Here we show that octaploid embryos can develop to the blastocyst stage in vitro, but all fetus impaired immediately after implantation. Our results indicate that cell lineage specification of octaploid embryo was disorganized. Furthermore, these octaploid embryos showed increased apoptosis as well as alterations in epigenetic modifications when compared with diploid embryos. Thus, our cumulative data provide cues for why mouse octaploid embryonic development is limited and its failed postimplantation development.
Introduction
Polyploidy, or whole genome multiplication (WGM), is ubiquitous among plants,Citation1,2 estimates of the frequency of polyploidy in angiosperms range from 30–80%.Citation3 Nevertheless, in mammals, polyploidy is generally incompatible with normal development,Citation4 as intricate genomic networks, imbalance of imprinting and sex chromosome dosage, which cause ploidy changes are deleterious and often carcinogenic in animals.Citation2,4
Mouse tetraploid (4n) embryos die at midgestation stage and spontaneously abort.Citation5 In chimeric mice produced from the combination of diploid (2n) and 4n embryos, the 4n cells are excluded from the epiblast at midgestation and contribute to the placental trophoblast.Citation6,7 Work from other groups also showed that tetraploid cells persist in embryonic tissues of chimeras produced by 2n and 4n embryo aggregation.Citation8,9 When 4n blastocysts devoid of the inner cell mass (ICM) are used for blastocyst injection, the resulting ESC mice are completely ESC-derived; however, when 4n blastocysts which have ICM are used, the ESC mice are 2n/4n chimeric.Citation10 Tetraploid-derived blastocyst embryos had very few Oct4-positive cells at the mid-blastocyst stage and the inner cell mass biomarkers Oct4, Sox2 and Klf4 were expressed at less than 10% of the level observed in diploid blastocysts.Citation11 Interestingly, Recent work discovered that expression of Oct4 is lost in the tetraploid expanded blastocysts, whereas Cdx2 is strongly expressed at this stage.Citation12 It was reported that Ccnb1 (cyclin B1), which was reduced by 3-fold in tetraploid embryos, appears to play a particularly important role in the cellular organization of the tetraploid blastocyst.Citation13 Cyclin B1 is regulatory protein involved in mitosis. Spindle microtubules also play a key role in mitotic events.Citation14 As structural components of the cell spindle microtubules are not only essential for the proper execution of most mitotic events,Citation15 but they also function in the mechanisms that control the timing of these events.Citation16
Autophagy and apoptosis are critical processes during embryo implantation development. Evidence exists regarding the role of the tumor suppressor protein p53, in rescuing tetraploid development in the mammalian epiblast. Tetraploidy in differentiating epiblast cells triggers p53-dependent cell cycle arrest and apoptosis.Citation17 Depending on the location of the p53 tumor suppressor protein, it plays an important role in regulating autophagy as well. When in the nuclear region, p53 acts as a transcription factor in order to activate Dram1 and Sestrin2 which activates autophagy.Citation18 Additionally, the autophagy gene, Atg5 is required for the engulfment of apoptotic cells during the morphogenesis of specific organs during late embryonic development.Citation19
While the processes governing tetraploid embryonic development are well studied, octaploid embryonic progression and mechanisms behind the observed octaploid developmental defects are still very poorly understood. Here we first generate octaploid embryos and follow their developmental dynamics in detail, in parallel with diploid and tetraploid embryos. We found that octaploid embryos can develop to blastocyst stage in vitro, but all fetus dead immediately after implantation. We further show that cell lineage specification, apoptosis, autophagy and epigenetic modifications are altered and are likely causes for the failure of octaploid embryonic development in mice.
Results
Mouse octaploid embryos failed to undergo postimplantation development
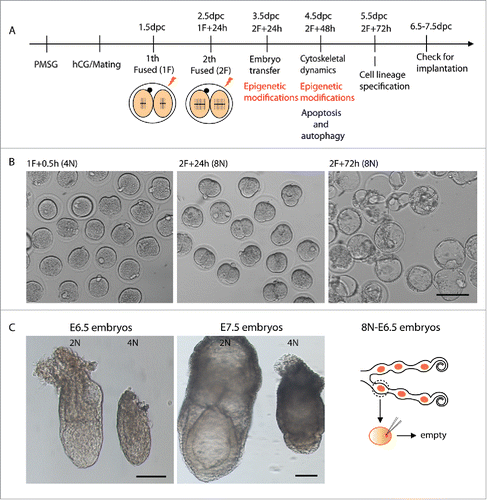
We generated mouse octaploid embryos and evaluated its in vitro/in vivo developmental potential. The detailed experimental procedures are shown in . Preimplantation development of the octaploid embryo was apparently normal in vitro. Octaploid embryos could develop to blastocyst stage at a rate of 74.9% which is similar to diploid (78.7%) and tetraploid (69.3%) embryos ( and Table S1). To further investigate octaploid embryonic development in vivo, we transferred 3.5 dpc octaploid embryos into 2.5 dpc pseudopregnant recipient females. The embryos were harvested at 6.5dpc and 7.5dpc. We found that epiblast-derived embryonic tissue is retarded in tetraploid embryos at E6.5 and often shows larger extra-embryonic tissue at E7.5.Citation5 However, octaploid embryos could not develop to E6.5 stage ( and Table S2). After embryonic transfer there were some implantation sites detected, but all embryos were absorbed. Our research demonstrates that octaploid embryos can develop up to the blastocyst stage, but they could not developed to epiblast stage. Thus, preimplantation events in octaploid embryo follow a “clock” that runs at the same pace as in diploid embryos, in agreement with earlier observations,Citation20,21 although, the precise mechanisms of octaploid embryonic developmental limitations are unknown.
Figure 1. Generation of mouse octaploid embryos and their in vitro/in vivo development. (A) Scheme for octaploid embryo generation and experimental design for mRNA and protein analysis of octaploid embryos. (B) Developmental stages/progression of octaploid embryos after first electrofusion 0.5 h and second electrofusion 24 h and 72 h. Scale bar, 100 μm. (C) Epiblast-derived embryonic tissue is retarded in tetraploid embryos at E6.5 and often shows larger extra-embryonic tissue in E7.5 compared to diploid embryos. Octaploid embryos could not develop to E6.5 stage. Scale bar, 100 μm.
Cell lineage specification is disorganized in octaploid embryos
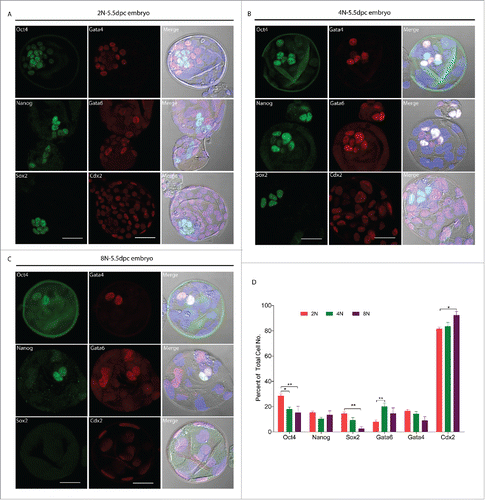
Immediately before implantation, the mouse blastocyst consists of 3 distinct cell groups: the epiblast, which is derived from the earlier inner cell mass (ICM), the primitive endoderm (PE) and the trophectoderm (TE).Citation22 We therefore wondered whether octaploid embryos have disordered cell lineage specification and if this might be reason for their reduced developmental potential. To test this hypothesis and in order to observe the dynamic changes during cell lineage specification of octaploid embryos, we determined the percentages of cells belonging to a specific lineage and conducted transcriptome analysis, at the end of preimplantation development in octaploid embryos. Our detailed examination through immunostaining of octaploid () embryos suggested that the percentages of inner cell mass biomarkers Oct4 and Sox2 positive cells were significantly reduced as compared to diploid ()embryos (15.3 ± 15.0 and 2.5 ± 9.9 vs. 28.5 ± 5.5 and 14.4 ± 4.3; p < 0.01; ). Surprisingly, we found the ICM cell numbers in octaploid embryos varied drastically, in comparison with diploid embryos. In tetraploid () embryos these results are similar to octaploids, the percentage of inner cell mass biomarker Oct4 was significantly reduced in tetraploid embryos (17.9 ± 5.3 vs. 28.5 ± 5.5; p < 0.05; ). To detect the status of primitive endoderm formation in octaploid embryos, we next tested the primitive endoderm-specific proteins Gata4 and Gata6, we found that Oct4 and Gata4 colocalized in the octaploid () nuclei, which does not occur in diploid () embryos. We also stained for the trophectoderm biomarker Cdx2, in octaploid () embryos the percentage of Cdx2 positive cells were significantly higher as compared to diploid embryos (92.3 ± 8.6 vs. 81.5 ± 4.1; p < 0.05; ). Real-time RT-PCR results showed that Nanog and Gata4 mRNA expression levels were significantly reduced in octaploid embryos (0.508 ± 0.113 and 0.551 ± 0.001 vs. 1.0; Fig. S1). These findings suggest that cell lineage specification of octaploid embryos were disorganized.
Figure 2. Cell lineage specification markers protein localization in mouse 2N, 4N and 8N blastocysts (E5.5). Immunofluorescent detection of Oct4, Nanog, Sox2, Gata4, Gata6 and Cdx2 in 2N, 4N and 8N embryos at the blastocyst Stage (E5.5) cultured in vitro. (A) Immunostaining for cell lineage specification markers in the 2N blastocyst. (B) The localization pattern of cell lineage specification markers in 4N blastocyst was similar to 2N. (C) In 8N blastocyst, Oct4 and Gata4 colocalized in nuclei and had very few Sox2 positive cells. Gray- DIC; Green- Oct4, Nanog, Sox2; Red- Gata4, Gata6, Cdx2; Blue- DAPI (4,6-diamidino-2-phenylindole) Scale bar, 40 μm. (D) Percent of different embryonic layer markers at total cell number after immunostaining in 2N, 4N and 8N blastocyst. For analysis of cell linage specification, a total of 30 embryos were analyzed in each group and performed at least 3 times each group. *Significantly different (p< 0.05); **significantly different (p < 0.01); ***significantly different (p < 0.001).
Epigenetic modification level changes in octaploid embryos
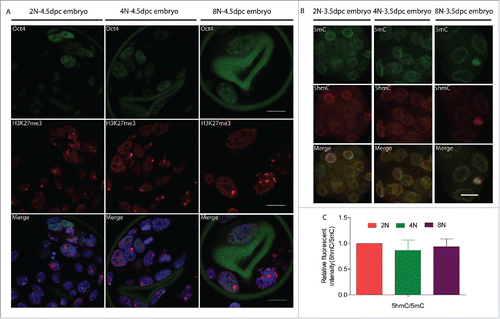
Mammalian X-chromosome inactivation (XCI) is a strategy to compensate for the imbalance in X-linked gene dosage between XX and XY, wherein one of the 2 X-chromosomes in females is transcriptionally inactivated.Citation23 One known epigenetic hallmark of the XCI is the histone H3 trimethyl-lysine 27 (H3K27me3) modification. H3K27me3 marks are present in differentiated cell subpopulation, whereas in inner cell mass and embryonic stem cell the 2 X-chromosomes in females are activated.Citation24 In our next analyses we examined the levels of H3K27me3 to determine whether this epigenetic modification was changed in octaploid embryos. As compared to control embryos, the H3K27me3 modification pattern in octaploid embryos was significantly different. While control blastocysts exhibit accumulation of H3K27me3 associated with the inactive X chromosome and tetraploid blastocysts have one to 2 foci of H3K27me3 per cell. Additionally, in control and tetraploid embryos, we can detect accumulation of H3K27me3 in Oct4 positive cells (Table S3). Whereas octaploid embryos show one to 4 H3K27me3 foci per nucleus and some Oct4 positive nuclei show abnormal H3K27me3 accumulation ( and Table S3). We next looked at overall DNA methylation levels by co-staining the embryos with antibodies against 5mC or 5hmC. The immunoflouresence data revealed that, at early blastocyst stage, the 5 hmC/5mC level were lower in tetraploid and octaploid embryos as compare with diploid controls (). From the above results, we conclude the epigenetic modifications are altered in octaploid embryos.
Figure 3. Epigenetic changes in 2N to 8N mouse embryos at the blastocyst stage (E4.5) cultured in vitro. Female 2N, 4N and 8N blastocyst exhibit different localization patterns of H3K27me3 foci associated with the inactive X chromosome. (A) Control blastocysts exhibit one H3K27me3 focus per cell; 4N blastocyst have one to 2 H3K27me3 foci; whereas octaploid embryos show one to 4 H3K27me3 foci including some Oct4 positive nuclei show abnormal H3K27me3 accumulation. Green- Oct4; Red- H3K27me3; Blue- DAPI (4,6-diamidino-2-phenylindole) Scale bar, 20 μm. (B) DNA methylation patterns in mouse 2N, 4N and 8N embryos. Immunofluorescence staining for 5mC and 5hmC in 2N, 4N and 8N embryos. Green, 5mC; red, 5hmC. Scale bar, 20 μm. (C) 5hmC/5mC level was lower in 4N and 8N embryos when compared with 2N control embryos. For analysis of epigenetic changes, a total of 30 embryos were analyzed in each group and performed at least 3 times each group.
Octaploid embryos exhibit increased apoptosis and autophagy levels
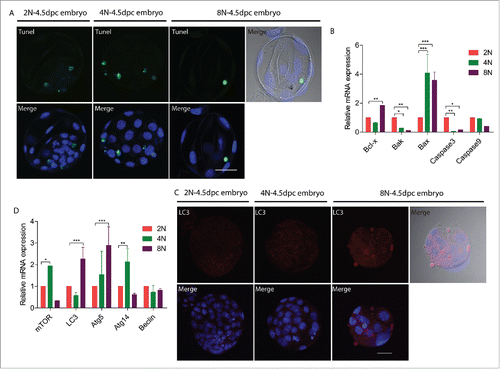
Tetraploidy in differentiating cells triggers p53-dependent cell cycle arrest and apoptosis. We wondered whether octaploid embryos exhibit apoptosis and autophagy. We next assessed markers for apoptosis and autophagy in octaploid embryos. Although Tunel staining for diploid, tetraploid and octaploid embryos show similar results (), the mRNA expression of apoptosis-related genes, Bcl-x and Bax were significantly higher in the octaploid embryos (1.85 ± 0.003 and 3.58 ± 0.57 vs. 1.0; p < 0.01; ). The process of apoptosis is often associated with autophagy. LC3 is one of the mammalian homologues of yeast ATG8, an important marker and effector of autophagy.Citation25 We also investigated LC3 level in octaploid embryos by immunofluorescent staining. This indicated that LC3 fluorescence level was increased in octaploid embryos (). Real-time RT-PCR results showed that Lc3 and Atg5 were significantly increased in octaploid embryos (2.26 ± 0.54 and 2.90 ± 0.86 vs. 1.0; p < 0.001; ). Thus, apoptosis as well as autophagy were induced in octaploid embryos.
Figure 4. Apoptosis and autophagy changes in 2N to 8N mouse embryos at the blastocyst stage (E4.5) cultured in vitro. (A) Tunel assay indicate that 2N, 4N and 8N embryos have same localization patterns. Gray- DIC; Green-Tunel; Blue- DAPI (4,6-diamidino-2-phenylindole), Scale bar, 40 μm. (B) Expression of apoptosis-related genes in 2N, 4N and 8N embryos. Bcl-x and Bax mRNA levels were significantly increased in 8N embryos. (C) Immunofluorescent staining for LC3 in 2N, 4N and 8N embryos. LC3 level was significantly higher in 8N embryos. Gray- DIC; Red- LC3; Blue- DAPI (4,6-diamidino-2-phenylindole). Scale bar, 40 μm. (D) Expression of autophagy-related genes in 2N, 4N and 8N embryos. The mRNA expression levels for Lc3 and Atg5 were significantly increased in 8N embryos. For analysis of apoptosis and autophagy changes, a total of 30 embryos were analyzed in each group and performed at least 3 times each group.*significantly different (p< 0.05); **significantly different (p < 0.01); ***significantly different (p < 0.001).
Discussion
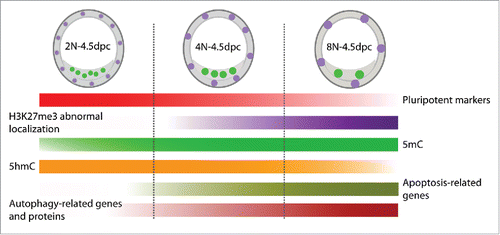
In this study, we investigated mouse preimplantation octaploid embryonic development and the possible mechanisms behind its failed postimplantation development. We found that cell lineage specification, epigenetic modifications, apoptosis and autophagy were changed in octaploid embryos (). Thus, these changes may be the possible reasons for impaired postimplantation development of mouse octaploid embryos.
Figure 5. A schematic showing dynamic changes in cell lineage specification, epigenetic modifications, apoptosis and autophagy in mouse 2N, 4N and 8N embryos.
Previous studies showed that octaploid mouse embryos produced by electrofusion polarize and cavitate at the same time as normal embryos and this suggests that the mechanisms underlying cell division, cavitation, and cortical polarization are not affected by changes in cellular size or ploidy.Citation20 In our study, we find that octaploid embryos can develop to the blastocyst stage, confirming the recently published results,Citation21 but all fetus impaired immediately after implantation. There is little information regarding mouse octaploid embryonic development. However, since tetraploid embryos were first produced more than 30 y ago Citation26,27 they are better studied, and provide the methodology for producing ES mice from embryonic stem cells lines.Citation28,29 It was recently demonstrated that the timing of compaction and attainment of the blastocyst stage in tetraploid was similar to those seen in normal fertilized diploid embryos,Citation12 we observe comparable dynamics for octaploid embryos, although the total cell number in the octaploid embryo was half of tetraploids.
After fertilization progression to gastrulation in embryos involves an ordered series of lineage specifications and axial asymmetries. It is well known that the preimplantation mammalian embryo is highly regulative and resistant to the loss or addition of cells brought about by experimental manipulations.Citation20 But it is not clear whether the lineage allocation in octaploid embryos is normal. To clarify this biological question, we followed lineage specification in octaploid embryos. Our experiments indicated that the percentages of ICM biomarkers Oct4 and Sox2 were significantly reduced in octaploid embryos. Primitive endoderm and epiblast are 2 lineages derived from the inner cell mass of the E3.5 blastocyst. Primitive endoderm and epiblast progenitors express the lineage-specific transcriptional factors Gata6/Gata4 and Nanog respectively.Citation30 Next, we assessed primitive endoderm formation in octaploid embryos and found that the percentage of Gata4 positive cells were reduced. Surprisingly, Oct4 and Gata4 colocalized in octaploid embryo nuclei, which indicates that primitive endoderm and epiblast specification was disordered. Consequently, the percentage of Cdx2 positive cells significantly increased. These findings suggest that cell lineage specification was disrupted during preimplantation octaploid embryonic development.
In female mammals, one of the 2 X chromosomes in each cell is transcriptionally silenced, through a process called X chromosome inactivation (XCI). The master regulator of this process is the X-inactive specific transcript (Xist) long non-coding RNA.Citation31 In mice, Xist expression is initiated around the 4-cell stage.Citation32,33 This expression pattern leads to the establishment of imprinted XCI in extra-embryonic tissues.Citation34 The inactive X chromosome is marked by the epigenetic modification histone H3 trimethyl-lysine 27 (H3K27me3). Xist and polycomb repressive complex2 mediate H3K27me3.Citation35 We observe that the H3K27me3 modification pattern in octaploid embryos is different from diploid embryos. Some octaploid embryos show 4 H3K27me3 foci and some Oct4 positive nuclei show abnormal H3K27me3 accumulation, which suggest that XCI process is abnormal in octaploid embryos.
DNA methylation is one of the best-characterized epigenetic modifications.Citation36 Genome-wide erasure of DNA cytosine-5 methylation (5mC) has been reported to occur in the paternal pronucleus in fertilized oocytes in an apparently replication-independent manner, 5-hydroxymethylcytosine (5hmC) also has been proposed as a potential intermediate in a replication dependant manner.Citation37 To investigate other potential causes for failed implantation, we examined the DNA methylation levels in octaploid embryos. Our results indicate that DNA methylation level in mouse octaploid embryos is lower compared to diploids and tetraploids. This suggests that DNA methylation levels may affect mouse octaploid embryonic development.
Published findings indicate that apoptosis-related genes increased in tetraploid embryos.Citation14 Furthermore, higher levels of p53 protein were detected in differentiated tetraploid embryonic stem cells compared to differentiated diploid embryonic stem cells, which led to higher expression of the Bax gene.Citation17 We also show that pro-apoptotic gene, Bax Citation38 expression level in tetraploid and octaploid embryos were significantly higher than in diploid embryos. Although, the expression level for the anti-apoptotic gene, Bcl-x was also increased in octaploid embryos. Unexpectedly, the apoptosis-related genes, Bak and Caspase-3 Citation38 expression levels were decreased drastically in tetraploid and octaploid embryos. Autophagy, along with apoptosis, is another well-known cell death mechanism, but it is now widely accepted as an adaptive response used for cell survival under conditions of stress.Citation18 Notably, our findings indicate that autophagy-related genes and proteins were significantly increased in octaploid embryos. Thus, apoptosis and autophagy induced in octaploid embryos may contribute to the reduced developmental potential.
In conclusion, our results indicate that octaploid embryos can develop to blastocyst stage, but they could not develop to the epiblast stage. We found that cell lineage specification, apoptosis, autophagy and epigenetic modification patterns were changed in octaploid embryos (). These alterations might be possible reasons for the developmental limitation of mouse octaploid embryos. Future studies of polyploidy must therefore strive to correlate whole genome and transcriptome changes with developmental changes to determine whether the genetic and epigenetic changes observed in polyploids are indeed adaptive.
Materials and methods
Ethic statement
Animal care and use were conducted in accordance with the guidelines of Nanjing Agricultural University, China. Mice were housed in a temperature-controlled room with proper darkness-light cycles, fed with a regular diet, and maintained under the care of the Laboratory Animal Unit, Nanjing Agricultural University, China. The mice were sacrificed by cervical dislocation. This study was specifically approved by the Institutional Animal Care and Use Committee, Nanjing Agricultural University, China.
Animals
Four-to-six-week-old female ICR mice (Beijing Vital River Laboratory Animal Co. Ltd, Beijing, China) were housed in a temperature-controlled (22 ± 2°C) room with 12-hour light cycle condition, fed a regular diet, and maintained under the care of the Animal Research Committee of Nanjing Agricultural University, China.
Mouse embryo harvesting
Female ICR mice were superovulated by injection of 5 IU of pregnant male serum gonadotropin (PMSG, NSH, China), followed 48 hr later by the injection of 5 IU of human chorionic gonadotropin (hCG, NSH, China), and then mated with male ICR mice. Two-cell embryos were collected by flushing oviducts with M2 medium (Sigma) 42–46 hr after hCG injection and cultured in KSOM medium (Millipore) under paraffin oil at 37°C in a 5% CO2 atmosphere.
Electrofusion parameters to generate tetraploid and octaploid embryos
To generate tetraploid embryos, the recovered 2-cell embryos were arrayed in a fusion chamber (1 mm) filed with 300 mM D-Sorbitol (Sigma) supplemented with 0.1 mM MgSO4·7H2O (Sigma), 0.05 mM CaCl2·2H2O (Sigma), and 3 mg/ml bovine serum albumin (Sigma). The key parameter for electrofusion was that a voltage differential of roughly 0.09–0.15 Vμm−1 be applied across the embryos. On the electrofusion device (BLS, CF-150), the “repeat” was set at 2. The square pulse voltage was 100 V and the pulse duration was 60 μs. After electrofusion, the embryos were washed 3 times in M2 medium (Sigma), and incubated in KSOM medium (Millipore) for 30 minutes and fusion usually took place within half an hour. When the tetraploid embryos developed to the 2-cell stage, electrofusion was performed again to produce octoploid embryos, using the same fusion conditions described above. The fused embryos were then cultured in KSOM medium (Millipore) under paraffin oil at 37°C in a 5% CO2 atmosphere.
Transfer of blastocyst-stage embryos and collection
On the day after the second fusion, the embryos should have reached the blastocyst stage (corresponding to 3.5 dpc of development) and were ready for transfer into pseudopregnant recipient females. Optimally, 8–10 embryos are transferred into each uterine horn of a 2.5 dpc recipients and then harvested at the embryonic stage 6.5–7.5 dpc.
Immunostaining of preimplantation embryos
Embryos were briefly washed with PBS and fixed in 4% paraformaldehyde in PBS for 20 min at room temperature. Embryos were permeabilized for 30 min with 1% BSA and 0.1% Triton X-100 in PBS. Antibody staining was carried out in the same buffer at 4°C overnight. The embryos were subsequently washed 3 times in 1% BSA, 0.1% Triton X-100 in PBS (5 min each wash), were incubated with secondary antibody for 1 h at room temperature in the dark, washed once for 5 min in 1% BSA, 0.1% Triton X-100 in PBS and twice for 5 min in PBS. The embryos were then mounted in Vectashield with DAPI (Vector Laboratories) and imaged using a Olympus FV1000 confocal microscope. Primary antibodies used for immunofluorescence including specific dilutions used are listed in Table S4. All secondary antibodies used were highly crossed adsorbed Alexa Fluor (Life technologies, 1:500).
Fluorescence intensity analysis
For the analysis of fluorescence intensity of 5 hmC and 5 mC, the embryos were mounted on the same glass slide, and the same scanning settings were used for sample scanning with a Olympus FV1000 confocal system. ImageJ software (NIH) was used to assess the fluorescence intensity levels. A region of interest (ROI) was defined for an embryo's chromosome, and the average fluorescence intensity per unit area within this ROI was determined. Independent measurements using identically sized ROIs were taken, and the average values of all measurements were used to determine the final average intensities for the 3 groups. A total of 30 oocytes were analyzed in each group.
TUNEL assay
Apoptosis was determined by the terminal deoxyribonucleotidyltransferase-mediated dUTP nick end labeling method. The detailed procedure was performed according to the protocol of the DeadEnd™ Fluorometric TUNEL Kit (Promega Corporation, Cat. No. G3250). After TUNEL reactions, embryos were mounted with Vectashield with DAPI (Vector Laboratories) and imaged using an Olympus FV1000 confocal microscope.
RNA purification and quantitative real time PCR
Total embryonic RNA was isolated with the RNeasy® Plus Mini Kit (Qiagen) and reverse transcribed into cDNA using the Reverse Transcription System (Promega) according to the manufacturer's instructions. Quantitative real-time PCR (qRT-PCR) was conducted using a PikoReal Real-Time PCR System (Thermo Scientific) and qRT-PCR reaction was performed with QuantiFast® SYBR® Green PCR kit (Qiagen). At least triplicate samples were assessed for each gene of interest, and GAPDH was used as a control gene. Relative expression levels were determined by the 2−△△Ct method. Primer sequences used are given in Table S5.
Statistical analysis
For each group, 3 replicates were done with results expressed as means ± SD. Statistical comparisons were made by ANOVA, followed by Duncan multiple comparisons test. A p-value of < 0.05 was considered significant.
Disclosure of potential conflicts of interest
The authors have no conflicts of interest to disclose.
Author contributions
B.J.W., S.Q.B., X.H.L and S.C.S. conceived and designed the experiments; B.J.W., S.Q.B., L.X.Z and C.C.Z. performed the experiments; B.J.W., S.Q.B., X.H.L and S.C.S. analyzed the data; Y.L.C. and M.Y.W. contributed reagents/materials/analysis tools; B.J.W., S.Q.B., X.H.L and S.C.S. wrote the paper.
KCCY_A_1252884_Supplementary_material.zip
Download Zip (56.7 KB)Acknowledgments
We thank Roopsha Sengupta for critical reading of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 31560335, 31622055 and 31571547), the Ministry of Science and Technology project of Inner Mongolia (No. 20121406 and 20130216) and the Program of Higher-level Talents of Inner Mongolia University (No. 135122), and the Fundamental Research Funds for the Central Universities (KJJQ201501, KJTZ201602, KJYQ201701), China.
References
- Renny-Byfield S, Wendel JF. Doubling down on genomes: polyploidy and crop plants. Am J Bot 2014; 101:1711-25; PMID:25090999; http://dx.doi.org/10.3732/ajb.1400119
- Chen ZJ. Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu Rev Plant Biol 2007; 58:377-406; PMID:17280525; http://dx.doi.org/10.1146/annurev.arplant.58.032806.103835
- Hegarty MJ, Hiscock SJ. Genomic clues to the evolutionary success of polyploid plants. Curr Biol 2008; 18:R435-44; PMID:18492478; http://dx.doi.org/10.1016/j.cub.2008.03.043
- Wertheim B, Beukeboom LW, van de Zande L. Polyploidy in animals: effects of gene expression on sex determination, evolution and ecology. Cytogenet Genome Res 2013; 140:256-69; PMID:23817224; http://dx.doi.org/10.1159/000351998
- Tarkowski AK, Witkowska A, Opas J. Development of cytochalasin in B-induced tetraploid and diploid/tetraploid mosaic mouse embryos. J Embryol Exp Morphol 1977; 41:47-64; PMID:591878
- Mackay GE, West JD. Fate of tetraploid cells in 4n<–>2n chimeric mouse blastocysts. Mech Dev 2005; 122:1266-81; PMID:16274964; http://dx.doi.org/10.1016/j.mod.2005.09.001
- Ishiguro N, Kano K, Yamamoto Y, Taniguchi K. Tetraploid cells of enhanced green fluorescent protein transgenic mice in tetraploid/diploid-chimeric embryos. J Reprod Dev 2005; 51:567-72; PMID:16034195; http://dx.doi.org/10.1262/jrd.17004
- Goto Y, Matsui J, Takagi N. Developmental potential of mouse tetraploid cells in diploid <–>tetraploid chimeric embryos. Int J Dev Biol 2002; 46:741-5; PMID:12216986
- Eakin GS, Hadjantonakis AK, Papaioannou VE, Behringer RR. Developmental potential and behavior of tetraploid cells in the mouse embryo. Dev Biol 2005; 288:150-9; PMID:16246322; http://dx.doi.org/10.1016/j.ydbio.2005.09.028
- Wen D, Saiz N, Rosenwaks Z, Hadjantonakis AK, Rafii S. Completely ES cell-derived mice produced by tetraploid complementation using inner cell mass (ICM) deficient blastocysts. PLoS One 2014; 9:e94730; PMID:24733255; http://dx.doi.org/10.1371/journal.pone.0094730
- Park MR, Hwang KC, Bui HT, Cho SG, Park C, Song H, Oh JW, Kim JH. Altered gene expression profiles in mouse tetraploid blastocysts. J Reprod Dev 2012; 58:344-52; PMID:22362217; http://dx.doi.org/10.1262/jrd.11-110M
- Park MR, Lee AR, Bui HT, Park C, Park KK, Cho SG, Song H, Kim JH, Nguyen VT. Chromosome remodeling and differentiation of tetraploid embryos during preimplantation development. Dev Dyn 2011; 240:1660-9; PMID:21547981; http://dx.doi.org/10.1002/dvdy.22653
- Kawaguchi J, Kano K, Naito K. Expression profiling of tetraploid mouse embryos in the developmental stages using a cDNA microarray analysis. J Reprod Dev 2009; 55:670-5; PMID:19789425; http://dx.doi.org/10.1262/jrd.09-127A
- Cadart C, Zlotek-Zlotkiewicz E, Le Berre M, Piel M, Matthews HK. Exploring the function of cell shape and size during mitosis. Dev Cell 2014; 29:159-69; PMID:24780736; http://dx.doi.org/10.1016/j.devcel.2014.04.009
- Avila J. Microtubule functions. Life Sci 1992; 50:327-34; PMID:1732704; http://dx.doi.org/10.1016/0024-3205(92)90433-P
- Sluder G. Role of spindle microtubules in the control of cell cycle timing. J Cell Biol 1979; 80:674-91; PMID:572367; http://dx.doi.org/10.1083/jcb.80.3.674
- Horii T, Yamamoto M, Morita S, Kimura M, Nagao Y, Hatada I. p53 suppresses tetraploid development in mice. Sci Rep 2015; 5:8907; PMID:25752699; http://dx.doi.org/10.1038/srep08907
- Maiuri MC, Malik SA, Morselli E, Kepp O, Criollo A, Mouchel PL, Carnuccio R, Kroemer G. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle 2009; 8:1571-6; PMID:19377293; http://dx.doi.org/10.4161/cc.8.10.8498
- Mizushima N. Autophagy: process and function. Genes Dev 2007; 21:2861-73; PMID:18006683; http://dx.doi.org/10.1101/gad.1599207
- Winkel GK, Nuccitelli R. Octaploid mouse embryos produced by electrofusion polarize and cavitate at the same time as normal embryos. Gamete Res 1989; 24:93-107; PMID:2591855; http://dx.doi.org/10.1002/mrd.1120240112
- Gu Y, Shen X, Zhou D, Wang Z, Zhang N, Shan Z, Jin L, Lei L. Selection and expression profiles of reference genes in mouse preimplantation embryos of different ploidies at various developmental stages. PLoS One 2014; 9:e98956; PMID:24927500; http://dx.doi.org/10.1371/journal.pone.0098956
- Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 2009; 136:701-13; PMID:19201946; http://dx.doi.org/10.1242/dev.017178
- Payer B, Lee JT. X chromosome dosage compensation: how mammals keep the balance. Annu Rev Genet 2008; 42:733-72; PMID:18729722; http://dx.doi.org/10.1146/annurev.genet.42.110807.091711
- Hong SH, Rampalli S, Lee JB, McNicol J, Collins T, Draper JS, Bhatia M. Cell fate potential of human pluripotent stem cells is encoded by histone modifications. Cell Stem Cell 2011; 9:24-36; PMID:21726831; http://dx.doi.org/10.1016/j.stem.2011.06.002
- Cherra SJ, 3rd, Kulich SM, Uechi G, Balasubramani M, Mountzouris J, Day BW, Chu CT. Regulation of the autophagy protein LC3 by phosphorylation. J Cell Biol 2010; 190:533-9; PMID:20713600; http://dx.doi.org/10.1083/jcb.201002108
- Modlinski JA. Transfer of embryonic nuclei to fertilised mouse eggs and development of tetraploid blastocysts. Nature 1978; 273:466-7; PMID:566383; http://dx.doi.org/10.1038/273466a0
- Modlinski JA. The fate of inner cell mass and trophectoderm nuclei transplanted to fertilized mouse eggs. Nature 1981; 292:342-3; PMID:7254330; http://dx.doi.org/10.1038/292342a0
- Nagy A, Gocza E, Diaz EM, Prideaux VR, Ivanyi E, Markkula M, Rossant J. Embryonic stem cells alone are able to support fetal development in the mouse. Development 1990; 110:815-21; PMID:2088722
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A 1993; 90:8424-8; PMID:8378314; http://dx.doi.org/10.1073/pnas.90.18.8424
- Yamanaka Y, Lanner F, Rossant J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development 2010; 137:715-24; PMID:20147376; http://dx.doi.org/10.1242/dev.043471
- Cerase A, Pintacuda G, Tattermusch A, Avner P. Xist localization and function: new insights from multiple levels. Genome Biol 2015; 16:166; PMID:26282267; http://dx.doi.org/10.1186/s13059-015-0733-y
- Augui S, Nora EP, Heard E. Regulation of X-chromosome inactivation by the X-inactivation centre. Nat Rev Genet 2011; 12:429-42; PMID:21587299; http://dx.doi.org/10.1038/nrg2987
- Nesterova TB, Barton SC, Surani MA, Brockdorff N. Loss of Xist imprinting in diploid parthenogenetic preimplantation embryos. Dev Biol 2001; 235:343-50; PMID:11437441; http://dx.doi.org/10.1006/dbio.2001.0295
- Takagi N, Sasaki M. Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature 1975; 256:640-2; PMID:1152998; http://dx.doi.org/10.1038/256640a0
- Calabrese JM, Sun W, Song L, Mugford JW, Williams L, Yee D, Starmer J, Mieczkowski P, Crawford GE, Magnuson T. Site-specific silencing of regulatory elements as a mechanism of X inactivation. Cell 2012; 151:951-63; PMID:23178118; http://dx.doi.org/10.1016/j.cell.2012.10.037
- Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010; 466:1129-33; PMID:20639862; http://dx.doi.org/10.1038/nature09303
- Iqbal K, Jin SG, Pfeifer GP, Szabo PE. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc Natl Acad Sci U S A 2011; 108:3642-7; PMID:21321204; http://dx.doi.org/10.1073/pnas.1014033108
- Sochalska M, Tuzlak S, Egle A, Villunger A. Lessons from gain- and loss-of-function models of pro-survival Bcl2 family proteins: implications for targeted therapy. FEBS J 2015; 282:834-49; PMID:25559680; http://dx.doi.org/10.1111/febs.13188
