G protein coupled receptors, or GPCRs, are the largest family of cell surface receptors with over 800 members identified in the human genome. These receptors are involved in nearly all aspects of physiology, transducing signals as diverse as light and odor along with paracrine, endocrine and neuronal signaling. Because of their cell-surface location and broad physiological roles they are an extremely important class of drug targets.
When these were first described, GPCRs were thought to be simple on/off switches. The magnitude of cellular response was assumed to be directly proportional to occupancy and there were therefore only 2 possible pharmacological interventions: mimic the natural ligand to switch the receptor on or block the binding of the natural ligand to turn the receptor off. If one wanted to reduce the hypertensive effect of catecholamines you would design a drug that blocks binding to the β1-adrenergic receptor; an antagonist. Similarly, if you wanted to provide analgesia via opioid receptors you would design a mimetic for the endogenous opioids; an agonist. To obtain a pharmaceutical with better clinical efficacy you would try and improve the affinity to the receptor while improving bioavailability.
As more pharmaceuticals targeting GPCRs were discovered, drugs with interesting pharmacologies began to appear. These included drugs that were never capable of eliciting the same maximal response as the natural ligand and drugs that suppressed baseline cellular activity; partial agonists and inverse agonists, respectively. These exemplify the concept of ligand efficacy. Parallel work by molecular pharmacologists and biochemists was taking place to understand the molecular basis of signal transduction. It became apparent that proteins are very dynamic, adopting a wide range of different tertiary structures of similar free energy.Citation1 For GPCRs, this conformational landscape included more than one “on” state, such that a partial agonist may bind to all the available receptors but each individual receptor was not capable of full effector coupling. In addition, the manner in which any ligand binds was inferred to be via conformational selection. Conformational selection means that prior to ligand binding the GPCR explores a range of conformational states, including a variety of “on” and “off” states. A full agonist will only bind with high affinity to a subset of the “on” states and will thus shift the equilibrium by effectively removing this receptor from the previous equilibrium. Similarly, an inverse agonist preferentially binds to a subset of “off” states and effectively shifts the equilibrium of receptor conformation away from any “on” states, thus reducing basal activity.Citation2 In a corollary to this, ligand-specific stabilization of distinct conformational ensembles can alter the pattern of effector engagement, an effect termed biased agonism.Citation3 These concepts are being actively pursued by pharmaceutical companies and are thought, at least in part, to underlie the different clinical effectiveness of apparently similar drugs acting at the same receptor.
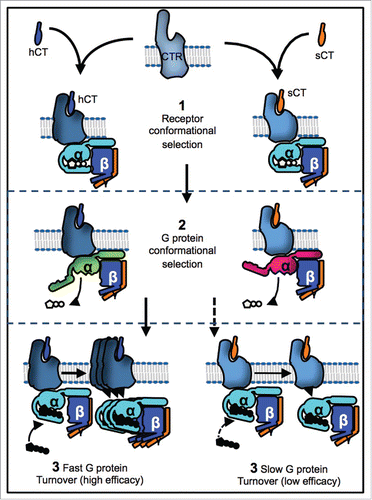
Figure 1. Ligand binding to GPCRs is characterized by conformational selection for the high affinity state an example of which is binding of human (hCT) and salmon (sCT) calcitonin agonists to the calcitonin receptor (CTR) that stabilizes different receptor conformations (1). We show that this conformational selection at the CTR extends to the G protein effector (2) whose conformation is differentially stabilized by the 2 agonists (green v's pink). This controls the rate at which G proteins turn over at the receptor leading to differential efficacy (3).
The fundamental role of the GPCR is to sense an external stimulus and transduce this to a cellular effector. In light of the above discussion it will be evident that the “on” states that are stabilized by agonist promoted conformational selection will be states with higher affinity for the effector. Thus effector and agonist have reciprocal effects with respect to each other's binding. The best-characterized GPCR effectors are the heterotrimeric G proteins. Upon GPCR activation these are recruited to the receptor, which then acts as a guanine nucleotide exchange factor to stimulate GDP release and GTP binding, with the GTP bound G protein being active and able to stimulate downstream signaling pathways. It was inferred that the magnitude of response these conformations could illicit was driven by the relative affinity these states had for the G protein effector.
We examined this paradigm for a prototypic class B GPCR, the calcitonin receptor.Citation4 We directly measured the micro-affinity “on” state of the ligand:receptor:G protein complex by both native PAGE and in vitro G protein BRET assays. We found the micro-affinity “on” state of the ligand:receptor:G protein complex differed for 2 agonists (salmon and human calcitonin) even though their ability to promote a cellular response via this G protein was identical. We therefore used native PAGE (including in-gel FRET), in vitro G protein BRET and GTP on-rate experiments to determine whether there were conformational differences in the bound G protein depending on agonist. All 3 lines of evidence supported a model where the conformation of the bound G protein was different depending on agonist. This implied that conformational selection extends beyond the GPCR to the effector. We found that, for the agonist with the lower promoted ligand:receptor:G protein complex affinity, the G protein conformation was such that it was more sensitive to disruption by GTP and the GTP association rate was faster. This faster GTP on-rate correlated with much shorter residence time for the G protein at the receptor when measured by TIRF microscopy. In a real-time whole-cell assay, to monitor the rate of production of the downstream cAMP signaling molecule, the “low” affinity agonist caused more rapid accumulation, consistent with biophysical observations and in contrast to expectations regarding the relationship between binding affinities and cellular efficacy (a summary of this model is shown in ). This, therefore, identifies that the key signal transduction step of effector coupling is one at which efficacy can be differentially regulated and complements recent work pointing to conformational selection on the less well-characterized GPCR effector β-arrestin.Citation5
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Frauenfelder H, Sligar SG, Wolynes PG. The energy landscapes and motions of proteins. Science 1991; 254:1598-603; PMID:1749933; http://dx.doi.org/10.1126/science.1749933
- Kim TH, Chung KY, Manglik A, Hansen AL, Dror RO, Mildorf TJ, Shaw DE, Kobilka BK, Prosser RS. The role of ligands on the equilibria between functional states of a G protein-coupled receptor. J Am Chem Soc 2013; 135:9465-74; PMID:23721409; http://dx.doi.org/10.1021/ja404305k
- Shonberg J, Lopez L, Scammells PJ, Christopoulos A, Capuano B, Lane JR. Biased agonism at G protein-coupled receptors: the promise and the challenges - a medicinal chemistry perspective. Med Res Rev 2014; 34:1286-330; PMID:24796277; http://dx.doi.org/10.1002/med.21318
- Furness SGB, Liang Y-L, Nowell CJ, Halls ML, Wookey PJ, Dal Maso E, Inoue A, Christopoulos A, Wootten D, Sexton PM. Ligand-dependent modulation of G protein conformation alters drug efficacy. Cell 2016; 167:739-749.e11; http://dx.doi.org/10.1016/j.cell.2016.09.021
- Lee M-H, Appleton KM, Strungs EG, Kwon JY, Morinelli TA, Peterson YK, Laporte SA, Luttrell LM. The conformational signature of β-arrestin2 predicts its trafficking and signalling functions. Nature 2016; 531:665-8; PMID:27007854; http://dx.doi.org/10.1038/nature17154
