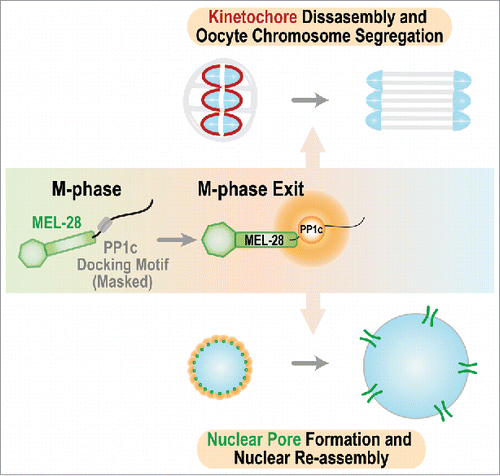
The dramatic transformation of cellular architecture and physiology that occurs during entry into M-phase of the cell cycle is catalyzed by protein kinases such as CDK1/Cyclin B, Polo-like kinase 1, and Aurora A/B. During this transformation, phosphorylation of multiple substrates leads to permeabilization and disassembly of the nuclear compartment, condensation of the replicated genome, assembly of kinetochores—the specialized protein structures on chromosomes that mediate microtubule interactions and act as signaling hubs—on centromeric chromatin, and formation of a bipolar spindle. During exit from M-phase, which begins coincident with separation of chromosomes on the spindle, this complex transformation must be reversed to complete cell division and form 2 daughter cells. This reversal, as well as proper entry and progression through M-phase, requires the action of phosphatases, most prominently of the PP2A and PP1 families. In a recent study,Citation1 we uncover an unexpected role for a nucleoporin in concentrating PP1 activity during M-phase exit and implicate this activity in disassembly of kinetochores and reassembly of functional nuclei ().
Nuclear disassembly at M-phase entry initiates with disassembly of nuclear pores, the large multi-subunit gateways embedded in the nuclear envelope that control traffic in and out of the nucleus during interphase. Pioneering work over a decade ago found that, following their removal from pores during M-phase entry, a subset of nucleoporins localize to the kinetochore regions of chromosomes. Kinetochore-localized nucleoporins include the Y-complex (also known as the Nup107/160 complex), a core structural scaffold of NPCs.Citation2 However, due to their long half-lives and critical role in NPC function during interphase, the role of the Y-complex nucleoporins at kinetochores has proven challenging to study.
Our recent study used RNAi-mediated depletion in a pre-formed C. elegans germline with several hundred precursor nuclei to bypass the essential role of Y-complex nucleoporins in nuclear compartmentalization and analyze their role in oocyte meiosis chromosome segregation. Use of penetrant RNAi and single-copy gene replacement demonstrated that a specific component of the Y-complex, MEL-28 (ELYS/AHCTF1 in vertebrates) is critical for meiotic chromosome segregation (a conclusion supported by an independent recent studyCitation3) and confirmed prior work that it is also essential for nuclear reassembly in the zygote. MEL-28ELYS is conserved across species that conduct open mitosis (in which the nuclear envelope is permeabilized or disassembled during chromosome segregation) and contains 2 common structural motifs of nuclear pore proteins: a β-propeller and an α-helical domain in its N-terminal half; the C-terminal half is poorly conserved and predicted to be unstructured.Citation4 The extreme C-terminus of MEL-28ELYS contains an AT-hook chromatin-binding domain that is thought to associate with decondensing chromatin during M-phase exit; the structured domain of MEL-28ELYS is proposed to then recruit other Y-complex components to initiate assembly of a nuclear pore in the reforming nuclear envelope.Citation5
Our effort to identify the mechanism by which MEL-28ELYS promoted meiotic segregation identified the functionally critical element to be a conserved bipartite docking motif for Protein Phosphatase 1 (PP1c) immediately following the structured N-terminal half. Depletion of MEL-28ELYS or replacement with a mutant unable to bind PP1c, resulted in a failure to segregate chromosomes in oocyte meiosis I, despite normal recruitment of the mutant MEL-28 to chromosomes, construction of a bipolar spindle and biochemical progression into anaphase. PP1c localization on anaphase chromosomes was greatly reduced in both the MEL-28 depletion and in the MEL-28 mutant that is unable to bind PP1c. Intriguingly, the MEL-28 bound PP1c acted during meiotic segregation by promoting disassembly of kinetochores that seems to be required to elongate the meiotic spindle and segregate homologous chromosomes. During mitosis, there is a significant increase in PP1c recruitment to chromosomes contemporaneously with anaphase onset, suggesting that MEL-28 may also contribute to chromosomal recruitment of PP1c during anaphase of mitotic divisions.
MEL-28 is required for both meiotic segregation and nuclear assembly in the C. elegans zygote where, following completion of meiosis, the maternal and paternal pronuclei replicate their genomes before their mixing in the first mitotic division. In the absence of MEL-28, pronuclei remain small and do not expand, leading to severely perturbed mitosis. Intriguingly, selectively disrupting PP1c docking on MEL-28ELYS also resulted in a severe nuclear assembly defect and this docking was required to enrich PP1c on the periphery of the assembling nuclei. These observations highlight PP1c docking as being critical for the function of MEL-28 in nuclear assembly. This finding leads us to suggest that MEL-28 provides a localized source of PP1c that promotes local dephosphorylation of nuclear pore proteins and potentially other substrates important to rebuild a functional nucleus. Support for this model in human cell lines comes from a recent report suggesting that ELYS and PP1c regulate the phosphorylation and nuclear envelope recruitment of Lamin B Receptor (LBR).Citation6
MEL-28 docking-dependent PP1c chromosomal localization is restricted to anaphase during meiosis I, and PP1c localization on mitotic chromosomes significantly increases upon anaphase onset (due to the severe nuclear assembly defect we were unable to test if this increase was due to docking on MEL-28). These observations lead us to propose that the docking site on MEL-28ELYS is cell cycle-regulated and becomes active for PP1c docking specifically during M-phase exit. Thus, our results lead to a model in which exposure of the PP1c docking site of MEL-28 generates a locally high concentration of phosphatase activity on kinetochores to promote their disassembly and potentially also on chromatin to promote rebuilding of nuclear pores and formation of a functional nucleus. The PP1c docking domain of MEL-28ELYS is conserved, despite poor overall conservation of the unstructured C-terminal half, which is stimulating us to now analyze the role of this nucleoporin-targeted phosphatase activity in mammalian cell division.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Hattersley N, Cheerambathur D, Moyle M, Stefanutti M, Richardson A, Lee KY, Dumont J, Oegema K, Desai A. A nucleoporin docks protein phosphatase 1 to direct meiotic chromosome segregation and nuclear assembly. Dev Cell 2016; 38:463-77; PMID:27623381; http://dx.doi.org/10.1016/j.devcel.2016.08.006
- Hoelz A, Debler EW, Blobel G. The structure of the nuclear pore complex. Annu Rev Biochem 2011; 80:613-43; PMID:21495847; http://dx.doi.org/10.1146/annurev-biochem-060109-151030
- Gómez-Saldivar G, Fernandez A, Hirano Y, Mauro M, Lai A, Ayuso C, Haraguchi T, Hiraoka Y, Piano F, Askjaer P. Identification of conserved MEL-28/ELYS domains with essential roles in nuclear assembly and chromosome segregation. PLoS Genet 2016; 12(6) e1006131; PMID:27341616; http://dx.doi.org/10.1371/journal.pgen.1006131
- Bilokapic S, Schwartz TU. Structural and functional studies of the 252kDa nucleoporin ELYS reveal distinct roles for its three tethered domains. Structure 2013; 4:572-80; PMID:23499022; http://dx.doi.org/10.1016/j.str.2013.02.006
- Schellhaus AK, De Magistris P, Antonin W. Nuclear reformation at the end of mitosis. J Mol Biol 2016; 428:1962-85; PMID:26423234; http://dx.doi.org/10.1016/j.jmb.2015.09.016
- Mimura Y, Takagi M, Clever M, Imamoto N. ELYS regulates the localization of LBR by modulating its phosphorylation state. J Cell Sci 2016; 129:4200-12; PMID:27802161; http://dx.doi.org/10.1242/jcs.190678