The pancreas can be subdivided into 2 distinct structures: the exocrine and the endocrine tissues. The exocrine compartment secretes and transports digestive enzymes to the duodenum, whereas the endocrine tissue is organized into small clusters of cells termed islets of Langerhans. The latter contain 5 different hormone-secreting cell subtypes: α-, β-, δ-, ϵ- and PP-cells producing glucagon, insulin, somatostatin, ghrelin and pancreatic polypeptide (PP), respectively. Among these different hormones, insulin acts to decrease blood glucose levels (upon any increase), whereas glucagon prevents a hypoglycemia.
Type 1 diabetes is an auto-immune disease characterized by the selective destruction of insulin-secreting β-cells leading to chronic hyperglycemia. The latter, when poorly managed, can result in severe complications, including cardiovascular complications, blindness or amputation. The constantly increasing number of diabetic patients associated with the highly restrictive aspects of current therapies (mainly daily exogenous insulin injections) call for the establishment of novel strategies. Most current research efforts therefore aim at finding way to (re)generate insulin-producing cells by mimicking β-cell (neo)genesis.
Toward this goal, we initially focused on Arx and Pax4, 2 transcription factors involved in endocrine cell genesis during embryonic development and mutually inhibiting each other's production. By means of multiple loss-of-function approaches using the mouse as a model, we demonstrated that Arx is required for α-cell differentiation, whereas Pax4 is required for β- and δ-cell genesis.Citation1 Interestingly, through the forced expression of Arx in adult β-cells, we provided evidences that adult β-cells were more plastic than previously expected, such cells being literally converted into α-like cells upon Arx misexpression.Citation2
Based on these findings and the apparent versatility of the endocrine cell identity, we wondered whether α-cells could also be turned into β-cells. We therefore generated animals allowing the forced expression of Pax4 in α-cells and animals permitting the specific loss of Arx expression in α-cells.Citation3-5 In both instances, a conversion of α-cells into β-like cells was indeed observed. Surprisingly, we also outlined continued processes of compensatory α-cell neogenesis, such cells being again turned into β-like cells upon Pax4 misexpression/Arx inactivation. Importantly, the β-like cells thereby generated were found to be functional and able to replace their endogenous counterparts in vivo.Citation3-5 Lastly, further worked provided evidences that the main trigger of the conversion of α-cells into β-like cells (and the ensuing cycle of β-like cell neogenesis) was the downregulation of Arx activity, Pax4 misexpression mainly acting to decrease Arx expression.Citation5
Even though promising, these results remained far from a putative medical application. This prompted us to seek for compounds inducing similar α-cell-mediated β-like cell neogenesis processes. In collaboration with S. Kubicek's team, we thereby identified 2 distinct compounds acting on the same signaling pathway.Citation6,7
GABA induces α-cell-mediated β-like cell neogenesis.
We first identified GABA (γ-aminobutyric acid), a well-know neurotransmitter also produced by pancreatic β-cells and acting to suppress glucagon secretion. Interestingly, upon long-term treatment of α-cells with GABA, we noted a significant decrease in the expression of Arx.Citation7 Considering the aforementioned crucial role of Arx downregulation in the α-to-β-like cell conversion, this compound thus appeared of interest. Accordingly, previous reports suggested that GABA administration to NOD mice (Non-Obese Diabetic, a murine model of type 1 diabetes) prevented hyperglycemia onset. We therefore treated wild-type animals with GABA for long durations and noticed a massive insulin+ cells hyperplasia and a proportional islet hypertrophy.Citation7 Furthermore, by means of lineage tracing analyses combined to immunohistochemical analyses, we demonstrated that α-cells were converted into insulin-secreting β-like cells upon GABA administration. As seen in the previously mentioned transgenic models, we observed a compensatory α-like cell neogenesis, such cells being converted once again into β-like cells as long as the animals were supplemented with GABA. This continuous cycle of neogenesis/conversion was found sufficient to counter several rounds of chemically-induced diabetes, indicative of repeated cycles of functional β-like cell neogenesis.Citation7 Concomitantly, our collaborator's results provided more insights into the mechanisms of the action of GABA in α-cells.
Artemether interacts with gephyrin to stimulate GABA signaling
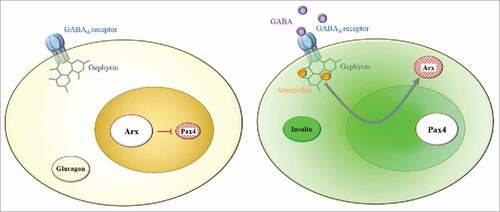
Artemether, classically used as an antimalarial treatment, was identified as a putative candidate triggering α-to-β-like cell conversion. Accordingly, this compound was found able to promote insulin secretion in α-cells in vitro.Citation6 Subsequently, a similar identity switch was confirmed in zebrafish, rodents and human cells. Interestingly, their results joined ours by establishing that arthemether acts through its interaction with gephyrin, a scaffold protein directly connected to the GABAA receptor, the GABAA receptor, present on α-cells (). Gephyrin potentiates GABA signaling and induces Arx translocation from the nucleus to the cytoplasm, thereby leading to its inactivation and the consequent conversion of α-cells into β-like cells.
Figure 1. Conversion of pancreatic α-cells into β-like cells upon GABA or arthemether administration. (Left) The GABAA receptor, expressed in all α-cells, interacts via its cytoplasmic portion with the scaffold protein gephyrin. In the α-cell nucleus, the transcription factor Arx represses the expression of Pax4 to maintain the α-cell identity. (Right) The GABA signaling pathway can be activated either by way of GABA's interaction with its receptor, or by arthemether's fixation on gephyrin. Both compounds eventually induce Arx inactivation (by down-regulation or translocation outside the nucleus), and thus the loss Pax4 repression, which triggers the conversion of α-cells into insulin-expressing β-like cells.
Conclusion
Altogether, these results identify GABA signaling activators and gephyrin stabilizers as 2 novel inducers β-cell neogenesis. Evidently, further work would be required to fully decipher the molecular mechanisms underlying the effects of GABA and Artemether. However, the previous pharmaceutical investigations of such compounds (Artemether is FDA-approved and GABA is GRAS - Generally Recognized As Safe) should simplify any prospective human application, including a potential synergistic combination of both compounds.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev 2003; 17:2591-603; PMID:14561778; http://dx.doi.org/10.1101/gad.269003
- Collombat P, Hecksher-Sorensen J, Krull J, Berger J, Riedel D, Herrera PL, Serup P, Mansouri A. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. J Clin Invest 2007; 117:961-70; PMID:17404619; http://dx.doi.org/10.1172/JCI29115
- Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell 2009; 138:449-62; PMID:19665969; http://dx.doi.org/10.1016/j.cell.2009.05.035
- Al-Hasani K, Pfeifer A, Courtney M, Ben-Othman N, Gjernes E, Vieira A, Druelle N, Avolio F, Ravassard P, Leuckx G, et al. Adult duct-lining cells can reprogram into beta-like cells able to counter repeated cycles of toxin-induced diabetes. Dev Cell 2013; 26:86-100; PMID:23810513; http://dx.doi.org/10.1016/j.devcel.2013.05.018
- Courtney M, Gjernes E, Druelle N, Ravaud C, Vieira A, Ben-Othman N, Pfeifer A, Avolio F, Leuckx G, Lacas-Gervais S, et al. The inactivation of Arx in pancreatic alpha-cells triggers their neogenesis and conversion into functional beta-like cells. PLoS genetics 2013; 9:e1003934; PMID:24204325; http://dx.doi.org/10.1371/journal.pgen.1003934
- Li J, Casteels T, Frogne T, Ingvorsen C, Honore C, Courtney M, Huber KV, Schmitner N, Kimmel RA, Romanov RA, et al. Artemisinins Target GABAA Receptor Signaling and Impair alpha Cell Identity. Cell 2017; 168:86-100 e15; PMID:27916275; http://dx.doi.org/10.1016/j.cell.2016.11.010
- Ben-Othman N, Vieira A, Courtney M, Record F, Gjernes E, Avolio F, Hadzic B, Druelle N, Napolitano T, Navarro-Sanz S, et al. Long-Term GABA Administration Induces Alpha Cell-Mediated Beta-like Cell Neogenesis. Cell 2017; 168:73-85 e11; PMID:27916274; http://dx.doi.org/10.1016/j.cell.2016.11.002
