After the discovery of the RET (rearranged during transfection) proto-oncogene functional studies revealed that RET is required for organogenesis and spermatogenesis.Citation1 Activation of RET leads to autophosphorylation on intracellular tyrosine residues and initiation of Ras/MAP kinase, PI3K/AKT, and phospholipase C-ɣ pathways that signal for cell proliferation, migration, and differentiation.Citation2 RET aberrations in diverse cancers were recently reported in Clinical Cancer Research from commercially available clinical grade next-generation sequencing (FoundationONE®) using data from 4,871 patients.Citation3 In addition to mutations in the RET proto-oncogene in medullary thyroid cancer, it also reported RET aberrations in multiple frequent cancer types including non-small cell lung cancer, uterine cancer, and rare cancers like hemagiopericytoma, pheochromocytoma fallopian tube cancer, and duodenal adenocarcinoma.Citation3 Several clinical trials with multi-kinase inhibitors that also target RET such as sunitinib, vandetanib, cabozantinib and lenvatinib have shown that RET is a druggable target in thyroid and non-small cell lung cancer. Not much data exists in other cancer types.
Data from TCGA, other large sequencing and big data Projects
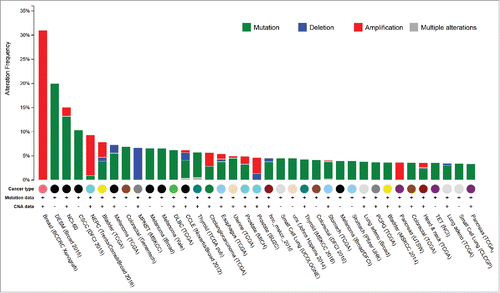
We analyzed RET aberration frequencies from the Cancer Genome Atlas (TCGA) and multiple other projects available on the c-Bio Portal.Citation4,Citation5 (, cancers with at least 3% RET alterations). Among 71 diverse cancers analyzed from 130 different studies including 2 cell line databases (NCI-60 and the Cancer Cell Line Encyclopedia) several diverse cancers harbored RET alterations including mutations, amplifications, and deletions. About 20% of desmoplastic melanomas (20 samples) harbored mutations in the RET geneCitation6 Cutaneous squamous cell carcinomas harbored RET mutations in 10% (3/29) of samples. Cutaneous melanomas also had RET alterations in 7.5% (5.6% mutation and 1.7% deletion) of tumors. In melanoma the Broad and Yale studies showed RET mutations in 6.6% of cases. In thyroid cancers, papillary thyroid tumors harbored a rate of 5.8% mutations, and anaplastic thyroid cancer showed 4.3%. In lung adenocarcinoma, the alteration rate in the Broad study was 3.8%, while in the TCGA study it was 3.5%. In the Genentech colorectal adenocarcinoma database the mutation rate was 6.9% and another study from DFCI showed a rate of 4.2%. The TCGA colon cancer data shows RET alterations in 3.6 percent of samples.
Figure 1. RET alteration frequencies across cancers. Mutation and copy number alteration frequency is displayed for cancers with at least three percent RET alteration (modified from cBioPortal).
RET inhibitors in the clinic
In addition to the several multi-kinase tyrosine kinase inhibitors such as sunitinib, vandetanib, cabozantinib, and lenvatinib that target RET, several novel highly selective RET inhibitors are in early stages of development (ClinicalTrials.gov Identifier:NCT03037385). Based on the frequency of RET alterations the first step in assessing the activity of these inhibitors in RET aberrant patients will be in the context of histology independent basket trials across multiple tumor types, in addition to RET altered medullary thyroid cancer and RET positive non-small cell lung cancer.
RET inhibitor resistance and co-occurring alterations
Given that resistance develops eventually to multi-kinase drugs that have RET inhibitor activity,Citation7 it would be worthwhile to evaluate the clinical responses to the highly selective RET inhibitors which possess more potent RET inhibitory activity in pre-clinical models than the current tyrosine kinase inhibitors. However, data from Kato et al show that RET aberrant patient samples also harbored co-alterations that can lead to tumorigenesis as well.Citation3 Among 88 patients, those included TP53-associated genes [e.g., MDM2, ATM, or TP53; 59.1% (52/88)], cell-cycle–associated genes [e.g., CDKN2A/B, CDK6, or RB1; 39.8% (35/88)], PI3K signaling pathway [e.g., PIK3CA, PTEN, AKT, or MTOR; 30.7% (27/88)], MAPK effectors [e.g., KRAS, NF1, or BRAF; 22.7% (20/88)], and other tyrosine kinase families [e.g., FGFR, EGFR, ERBB2, ALK, or KIT; 21.6% (19/88)]3. Hence, customized combinatorial therapiesCitation7 may need to be developed to target multiple alterations for overcoming the innate and acquired resistance for more effective therapies, toward precision oncology in RET aberrant tumors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev 2005; 16:441-67; PMID:15982921; http://dx.doi.org/10.1016/j.cytogfr.2005.05.010
- Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer 2014; 14:173-86; PMID:24561444; http://dx.doi.org/10.1038/nrc3680
- Kato S, Subbiah V, Marchlik E, Elkin SK, Carter JL, Kurzrock R. RET Aberrations in Diverse Cancers: Next-Generation Sequencing of 4,871 Patients. Clin Cancer Res 2016; 23(8):1988-1997; PMID:27683183; http://dx.doi.org/10.1158/1078-0432.CCR-16-1679
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6:pl1; PMID:23550210; http://dx.doi.org/10.1126/scisignal.2004088
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2:401-4; PMID:22588877; http://dx.doi.org/10.1158/2159-8290.CD-12-0095
- Shain AH, Garrido M, Botton T, Talevich E, Yeh I, Sanborn JZ, Chung J, Wang NJ, Kakavand H, Mann GJ, et al. Exome sequencing of desmoplastic melanoma identifies recurrent NFKBIE promoter mutations and diverse activating mutations in the MAPK pathway. Nat Genet 2015; 47:1194-9; PMID:26343386; http://dx.doi.org/10.1038/ng.3382
- Subbiah V, Berry J, Roxas M, Guha-Thakurta N, Subbiah IM, Ali SM, McMahon C, Miller V, Cascone T, Pai S, et al. Systemic and CNS activity of the RET inhibitor vandetanib combined with the mTOR inhibitor everolimus in KIF5B-RET re-arranged non-small cell lung cancer with brain metastases. Lung Cancer 2015; 89:76-9; PMID:25982012; http://dx.doi.org/10.1016/j.lungcan.2015.04.004
