ABSTRACT
William B. Coley developed bacterial therapy of cancer more than 100 years ago and had clinical success. James Ewing, a very famous cancer pathologist for whom the Ewing sarcoma is named, was Coley's boss at Memorial Hospital in New York and terminated Coley's bacterial therapy of cancer. A tumor from a patient with soft-tissue Ewing's sarcoma, who failed doxorubicin (DOX) therapy, was previously implanted in nude mice to establish a patient-derived orthotopic xenograft (PDOX) model. In the present study, the Ewing's sarcoma PDOX was treated with tumor-targeting S. typhimurium A1-R expressing green fluorescent (GFP), alone and in combination with DOX. S. typhimurium A1-R-GFP was detected in the tumors after intratumor (i.t.) or intravenous (i.v.) injection. The combination of S. typhimurium A1-R and DOX significantly reduced tumor weight (37.8 ± 15.6 mg) compared to the untreated control (73.8 ± 10.1 mg, P < 0.01). S. typhimurium A1-R monotherapy-treated tumors tended to be smaller (50.9 ± 17.8 mg, P = 0.051). DOX monotherapy did not show efficacy (66.3 ± 26.4 mg, P = 0.82), as was the case with the patient. The PDOX model faithfully replicated the DOX resistance the Ewing's sarcoma had in the patient. S. typhimurium A1-R converted the Ewing's sarcoma from DOX resistant to sensitive. One can only wonder how bacterial therapy and immunotherapy of cancer would have developed over the past 80 years if Ewing did not stop Coley.
Introduction
Ewing's sarcoma is a recalcitrant malignancy that usually occurs in adolescence and young adulthood. Multimodal treatment for localized Ewing's sarcoma has improved the 5-year survival to 65–70%. However, patients with metastatic disease have a 30% of 5-year survival rate.Citation1 Therefore, development of effective treatment for Ewing's sarcoma is needed.
William B. Coley developed bacterial therapy of cancer more than 100-years ago and had clinical success.Citation2,3
James Ewing was a very famous cancer pathologist for whom the Ewing sarcoma is named, was a bitter opponent of Coley's work at Memorial Hospital in New York and finally ended Coley's career and bacterial therapy of cancer.
In the current century, bacterial therapy is making a strong come back to enhance immunotherapy of cancer.Citation3 The tumor-targeting Salmonella typhimurium A1-R (S. typhimurium A1-R), developed by our laboratoryCitation4,5 is auxotrophic for Leu-Arg, which prevents it from mounting a continuous infection in normal tissues. S. typhimurium A1-R was effective against primary and metastatic tumors as monotherapy in nude mouse models of major cancers,Citation3 including prostate,Citation4,6 breast,Citation7-9 lung,Citation10,11 pancreatic,Citation12-16 ovarian,Citation17-18 stomach,Citation19 and cervical cancer,Citation20 as well as sarcoma cell linesCitation21-24 and glioma,Citation3,25 all of which are highly aggressive tumor models. In addition, S. typhimurium A1-R was effective against patient-derived orthotopic models of pancreatic cancer,Citation15,16 sarcomaCitation24,26-29 and melanoma.Citation30-32
A tumor from a patient with soft-tissue Ewing's sarcoma was previously established as a patient-derived orthotopic xenograft (PDOX) model.Citation28 In the present study, we observed that S. typhimurium A1-R converted the Ewing's sarcoma from doxorubicin (DOX)-resistant to DOX-sensitive.
Results and discussion
Ewing's sarcoma PDOX tumor recapitulates the original patient tumor
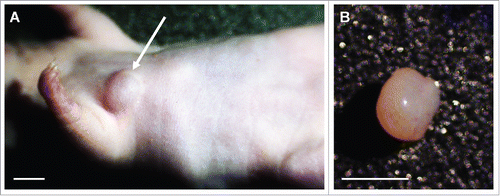
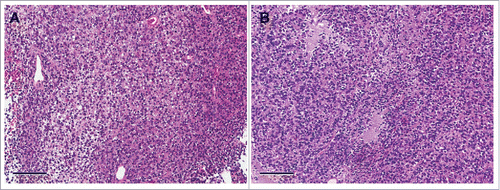
Four weeks after orthotopic implantation, the Ewing's sarcoma PDOX tumor grew to more than 60 mm3 in the right chest wall of nude mice (). Histologically, the patient's tumor demonstrated an infiltrative proliferation of small round blue cells. Cancer cells showed round-to-ovoid hyperchromatic nuclei, and scanty eosinophilic-to-clear cytoplasm (). Histology of the PDOX tumor had a similar morphologic appearance to the original patient tumor ().
S. typhimurium A1-R-GFP was detected in treated PDOX tumors
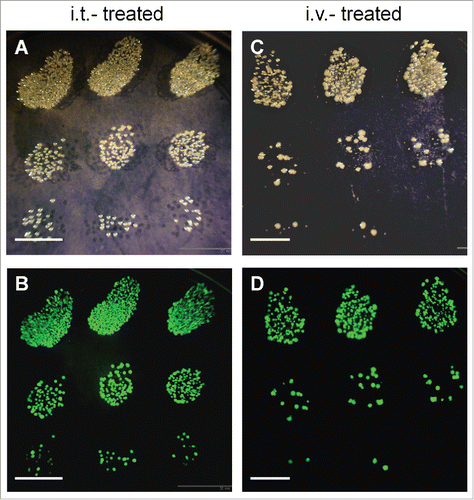
GFP-expressing S. typhimurium A1-R was detected in tumors on the next day after the 2nd i.t. injection () or i.v. injection of S. typhimurium A1-R-GFP (). More S. typhimurium A1-R-GFP was detected in the tumor after i.t. injection than i.v. injection, perhaps because the bacteria were directly injected. However, even with i.v. injection, a large amount of bacteria targeted the tumor from the circulation.
Figure 3. Bacterial culture from S. typhimurium A1-R-treated Ewing's sarcoma PDOX. Bright field (A, C) and fluorescence (B, D) images of GFP-expressing S. typhimurium A1-R colonies cultured in LB-agar. Administered S. typhimurium either by i.t. (A, B) or i.v. (C, D) was detected in treated tumors 24 hours after the second injection of S. typhimurium A1-R (day 9). Scale bars: 10 mm. GFP, green fluorescent protein. OV100 imaging.
S. typhimurium A1-R (i.t. and i.v.) inhibited tumor growth in the Ewing's sarcoma PDOX
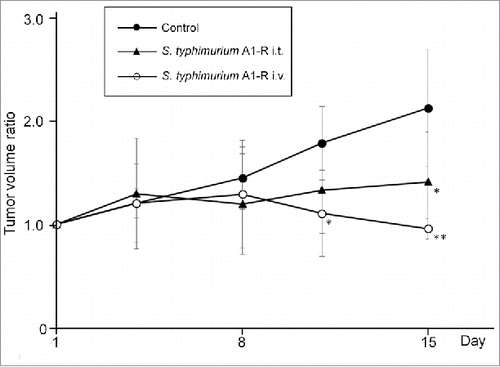
The tumor doubling time of the Ewing's sarcoma PDOX was 12.7 days. Both administrative routes (i.t. and i.v.) of S. typhimurium A1-R inhibited tumor growth (). A PDOX was previously established in the right chest wall of nude mice which corresponded to the origin of the tumor in the patient. Eighteen PDOX mice were randomized into three groups when tumor volume reached 60 mm3: untreated control; S. typhimurium A1-R intratumor administration (i.t., 5 × 107 CFU/25 µl, day 1 and 8), and S. typhimurium A1-R intravenous administration (i.v., 5 × 107 CFU/100 µl, day 1 and 8). Tumor growth was significantly suppressed in both of the S. typhimurium-treated groups. However, S. typhimurium A1-R i.v. was more effective than i.t. injection.
Figure 4. S. typhimurium A1-R administered i.v. or i.t. on the Ewing's sarcoma PDOX Line graph shows tumor volume ratio. S. typhimurium A1-R was administered i.v. or i.t. Please see the Materials and Methods for doses and schedules. *P < 0.05 and **P < 0.01 compared to control. Error bars: ± 1 SD. i.t., intratumoral injection; i.v., intravenous injection.
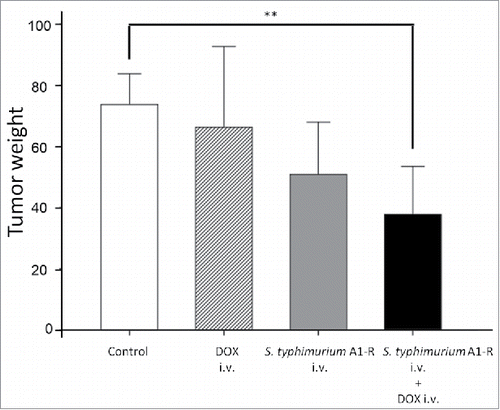
S. typhimurium A1-R i.v. increased the efficacy of doxorubicin in the Ewing's sarcoma PDOX model
S. typhimurium A1-R i.v. in combination with DOX, was then tested. Twenty PDOX mice were prepared as described above and randomized into the following groups; G1, untreated control; G2, DOX (i.v., 3 mg/kg, day 1 and 8); G3, S. typhimurium A1-R (i.v., 5 × 107 CFU/100 µl, day 1 and 8); G4, combination of S. typhimurium A1-R (i.v., 5 × 107 CFU/100 µl, day 1 and 8); and DOX (i.v., 3 mg/kg, day 4 and 11).
Only the combination of S. typhimurium A1-R and DOX significantly reduced tumor weight (37.8 μ ± 15.6 mg) compared to the untreated control (73.8 ± 10.1 mg, P < 0.01). S. typhimurium A1-R monotherapy-treated tumors tended to be smaller (50.9 ± 17.8 mg, P = 0.051). DOX monotherapy did not show efficacy (66.3 ± 26.4 mg, P = 0.82), as was the case with the patient (). Mouse body weight was not significantly different between groups at any time point.
Figure 5. Combination therapy of intravenous S. typhimurium A1-R (i.v.) combined with doxorubicin in the Ewing's sarcoma PDOX model. Bar graphs show resected tumor weight in each group. Please see the Materials and Methods for doses and schedules.
Bacterial therapy has potential side effects, even though they were not observed in the present study. Such side effects could include infection outside the tumor and potential unwanted immunological reaction.
Previously-developed concepts and strategies of highly selective tumor targeting can take advantage of molecular targeting of tumors, including tissue-selective therapy which focuses on unique differences between normal and tumor tissues.Citation33-38
The aim of the present study was to develop S. typhimurium A1-R treatment for an Ewing's sarcoma using different administrative routes and in combination with standard first-line DOX.
Conclusions
The present study has demonstrated the potential of S. typhimurium A1-R to be an effective treatment as both monotherapy and combination with first-line therapy for Ewing's sarcoma. Ironically, bacterial therapy was highly effective on a PDOX model of Ewing's sarcoma, demonstrating that the very treatment that was halted by Ewing was found more than 80 years later to be highly effective on the sarcoma named for him. One can only wonder how bacterial therapy and immunotherapy of cancer would have developed over the past 80 years if Ewing did not stop Coley.
Materials and methods
Mice
Athymic nu/nu nude mice (AntiCancer Inc., San Diego, CA), 4–6 weeks old, were used in this study under Assurance Number A3873-1. The animals were fed an autoclaved laboratory rodent diet.Citation28 Animals were anesthetized by subcutaneous injection of a 0.02 ml solution of 25 mg/kg ketamine, 19 mg/kg xylazine, and 0.60 mg/kg acepromazine maleate. Animals were housed with no more than 5 per cage and housed in a barrier facility on a high efficiency particulate arrestance (HEPA)-filtered rack under standard conditions of 12-hour light/dark cycles.
Patient-derived Ewing's sarcoma tumor
The female 46-year-old patient was previously diagnosed with Ewing's sarcoma in the right chest wall and received neoadjuvant chemotherapy including DOX. Then the tumor was resected by JY in the Department of Surgery, University of California, Los Angeles (UCLA). Written informed consent was obtained from the patient, and the Institutional Review Board (IRB) of UCLA approved this experiment.Citation28
Establishment of a PDOX model of Ewing's sarcoma
Our laboratory pioneered the patient-derived orthotopic xenograft (PDOX) nude mouse model with the technique of surgical orthotopic implantation (SOI), including pancreatic,Citation16,39-41 breast,Citation42 ovarian,Citation43 lung,Citation44 cervical,Citation45 colon,Citation46-48 stomach,Citation49 sarcoma,Citation26-28,50 and melanoma.Citation30-32
A Ewing's sarcoma PDOX was previously established on the right chest wall where a single tumor fragment was implanted orthotopically into the layer between pectoral muscle and intercostal muscle in nude mice.Citation28
Preparation of S. typhimurium A1-R
GFP-expressing S. typhimurium A1-R bacteria (AntiCancer Inc.,) were grown overnight on LB medium (Fisher Sci., Hanover Park, IL, USA) and then diluted 1:10 in LB medium. Bacteria were harvested at late-log phase, washed with PBS, and then diluted in PBS.Citation4,6,7
Efficacy of S. typhimurium A1-R i.v. vs. i.t. on the Ewing's sarcoma PDOX
PDOX tumors (n = 18) were divided into 3 groups when tumor volume reached 60 mm3: untreated control (n = 6); S. typhimurium A1-R (n = 6, i.t., 5 × 107 CFU/25 µl, day 1 and 8); S. typhimurium A1-R (n = 6, i.v., 5 × 107 CFU/100 µl, day 1 and 8). The mice were followed up until day 15. Tumor length, width, and mouse body weight were measured twice a week. Tumor volume was calculated by the following formula: Tumor volume (mm3) = length (mm) × width (mm) × width (mm) × 1/2. Tumor volume ratio was defined as the ratio of volume on each measurement day relative to day 0.
Efficacy of S. typhimurium A1-R in combination with DOX on the Ewing's sarcoma PDOX
PDOX tumors (n = 20) were divided into following 4 groups when the tumor volume reached 60 mm3: G1, untreated control (n = 5); G2; DOX (n = 5; i.v., 3 mg/kg, day 1 and 8); G3, S. typhimurium A1-R (n = 5, i.v., 5 × 107 CFU/100 µl, day 1 and 8); and G4; combination of S. typhimurium A1-R (n = 5, i.v., 5 × 107 CFU/100 µl, day 1 and 8) and DOX (n = 5, i.v., 3 mg/kg, day 4 and 11). Mice were sacrificed and tumors were removed on day 15 for weight determination. Tumor growth measurement and evaluation was performed as described above.
Histological analysis
Fresh tumor samples were fixed in 10% formalin and embedded in paraffin before sectioning and staining. Tissue sections (5 μm) were deparaffinized in xylene and rehydrated in an ethanol series. Hematoxylin and eosin (H&E) staining was performed according to standard protocol. Histological examination was performed with a BHS system microscope (Olympus Corp., Tokyo, Japan). Images were acquired with INFINITY ANALYZE software (Lumenera Corporation, Ottawa, Canada).Citation28
Bacterial culture from tumor tissue
To identify S. typhimurium A1-R in treated tumors, two Ewing's sarcoma PDOX mice were treated with S. typhimurium A1-R i.t. or i.v. These mice were sacrificed and tumors were resected on day 9 to culture bacteria. After removal of tumors from PDOX mice, the tumor specimens were homogenized and suspended in PBS (phosphate-buffered saline, Corning, New York, NY). The suspension was serially diluted, then cultured in LB agar for 12 hours. GFP-expressing colonies of S. typhimurium A1-R were detected with the OV100 Small Animal Imaging System (Olympus, Tokyo, Japan).Citation51
Statistical analysis
SPSS statistics version 21.0 was used for all statistical analyses (IBM, New York City, NY, USA). Significant differences for continuous variables were determined using the Student's t-test. Both line graphs and bar graph express average and error bar show standard deviation (SD). A probability value of P was calculated between control groups and each treatment group. P ≤ 0.05 was considered statistically significant.
Dedication
This paper is dedicated to the memory of A. R. Moossa, MD and Sun Lee, MD.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by National Cancer Institute grant number CA213649 and JSPS KAKENHI grant numbers 26830081 to YH, 26462070 to IE and 24592009 to KT.
References
- Gaspar N, Hawkins DS, Dirksen U, Lewis IJ, Ferrari S, Le Deley MC, Kovar H, Grimer R, Whelan J, Claude L, et al. Ewing Sarcoma: Current management and future approaches through collaboration. J Clin Oncol 2015; 33:3036-3046; PMID:26304893; https://doi.org/10.1200/JCO.2014.59.5256
- Nauts HC, Fowler GA, Bogatko FH. A review of the influence of bacterial infection and of bacterial products (Coley's toxins) on malignant tumors in man; a critical analysis of 30 inoperable cases treated by Coley's mixed toxins, in which diagnosis was confirmed by microscopic examination selected for special study. Acta Med Scand Suppl 1953; 276:1-103.
- Hoffman RM, editor. Bacterial Therapy of Cancer: Methods and Protocols. Methods in Molecular Biology 1409. Walker, John M., series ed. New York: Humana Press (Springer Science+Business Media); 2016.
- Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, Xu M, Penman S, Hoffman RM. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci USA 2005; 102:755-60; PMID:15644448; https://doi.org/10.1073/pnas.0408422102
- Zhang Y, Zhang N, Zhao M, Hoffman RM. Comparison of the selective targeting efficacy of Salmonella typhimurium A1-R and VNP20009 on the Lewis lung carcinoma in nude mice. Oncotarget 2015; 6:14625-31; PMID:25714030; https://doi.org/10.18632/oncotarget.3342
- Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci USA 2007; 104:10170-74; PMID:17548809; https://doi.org/10.1073/pnas.0703867104
- Zhao M, Yang M, Ma H, Li X, Tan X, Li S, Yang Z, Hoffman RM. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res 2006; 66:7647-52; PMID:16885365; https://doi.org/10.1158/0008-5472.CAN-06-0716
- Zhang Y, Tome Y, Suetsugu A, Zhang L, Zhang N, Hoffman RM, Zhao M. Determination of the optimal route of administration of Salmonella typhimurium A1-R to target breast cancer in nude mice. Anticancer Res 2012; 32:2501-08; PMID:22753706
- Zhang Y, Miwa S, Zhang N, Hoffman RM, Zhao M. Tumor-targeting Salmonella typhimurium A1-R arrests growth of breast-cancer brain metastasis. Oncotarget 2015; 6:2615-22; PMID:25575815; https://doi.org/10.18632/oncotarget.2811
- Uchugonova A, Zhao M, Zhang Y, Weinigel M, König K, Hoffman RM. Cancer-cell killing by engineered Salmonella imaged by multiphoton tomography in live mice. Anticancer Res 2012; 32:4331-7; PMID:23060555
- Liu F, Zhang L, Hoffman RM, Zhao M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumor vascularity. Cell Cycle 2010; 9:4518-24; PMID:21135579; https://doi.org/10.4161/cc.9.22.13744
- Nagakura C, Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Bouvet M, Hoffman RM. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res 2009; 29:1873-8; PMID:19528442
- Yam C, Zhao M, Hayashi K, Ma H, Kishimoto H, McElroy M, Bouvet M, Hoffman RM. Monotherapy with a tumor-targeting mutant of S. typhimurium inhibits liver metastasis in a mouse model of pancreatic cancer. J Surg Res 2010; 164:248-55; PMID:19766244; https://doi.org/10.1016/j.jss.2009.02.023
- Hiroshima Y, Zhao M, Zhang Y, Maawy A, Hassanein MK, Uehara F, Miwa S, Yano S, Momiyama M, Suetsugu A, et al. Comparison of efficacy of Salmonella typhimurium A1-R and chemotherapy on stem-like and non-stem human pancreatic cancer cells. Cell Cycle 2013; 12:2774-80; PMID:23966167; https://doi.org/10.4161/cc.25872
- Hiroshima Y, Zhao M, Maawy A, Zhang Y, Katz MH, Fleming JB, Uehara F, Miwa S, Yano S, Momiyama M, et al. Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX). J Cell Biochem 2014; 115:1254-61; PMID:24435915; https://doi.org/10.1002/jcb.24769
- Hiroshima Y, Zhang Y, Murakami T, Maawy AA, Miwa S, Yamamoto M, Yano S, Sato S, Momiyama M, Mori R, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograph (PDOX) and cell line mouse models. Oncotarget 2014; 5:12346-357; PMID:25402324; https://doi.org/10.18632/oncotarget.2641
- Matsumoto Y, Miwa S, Zhang Y, Hiroshima Y, Yano S, Uehara F, Yamamoto M, Toneri M, Bouvet M, Matsubara H, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R on nude mouse models of metastatic and disseminated human ovarian cancer. J Cell Biochem 2014; 115:1996-2003; PMID:24924355
- Matsumoto Y, Miwa S, Zhang Y, Zhao M, Yano S, Uehara F, Yamamoto M, Hiroshima Y, Toneri M, Bouvet M, et al. Intraperitoneal administration of tumor-targeting Salmonella typhimurium A1-R inhibits disseminated human ovarian cancer and extends survival in nude mice. Oncotarget 2015; 6:11369-77; PMID:25957417; https://doi.org/10.18632/oncotarget.3607
- Yano S, Zhang Y, Zhao M, Hiroshima Y, Miwa S, Uehara F, Kishimoto H, Tazawa H, Bouvet M, Fujiwara T et al. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell Cycle 2014; 13:3958-63; PMID:25483077; https://doi.org/10.4161/15384101.2014.964115
- Hiroshima Y, Zhang Y, Zhao M, Zhang N, Murakami T, Maawy A, Mii S, Uehara F, Yamamoto M, Miwa S, et al. Tumor-targeting Salmonella typhimurium A1-R in combination with Trastuzumab eradicates HER-2-positive cervical cancer cells in patient-derived mouse models. PLoS One 2015; 10:e0120358; PMID:26047477; https://doi.org/10.1371/journal.pone.0120358
- Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Hoffman RM. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J Cell Biochem 2009; 106:992-998; PMID:19199339; https://doi.org/10.1002/jcb.22078
- Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Kishimoto H, Bouvet M, Hoffman RM. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhimurium. Cell Cycle 2009; 8:870-5; PMID:19221501; https://doi.org/10.4161/cc.8.6.7891
- Miwa S, Zhang Y, Baek K-E, Uehara F, Yano S, Yamamoto M, Hiroshima Y, Matsumoto Y, Kimura H, Hayashi K, et al. Inhibition of spontaneous and experimental lung metastasis of soft-tissue sarcoma by tumor-targeting Salmonella typhimurium A1-R. Oncotarget 2014; 5:12849-61; PMID:25528763; https://doi.org/10.18632/oncotarget.2561
- Murakami T, DeLong J, Eilber FC, Zhao M, Zhang Y, Zhang N, Singh A, Russell T, Deng S, Reynoso J, et al. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft PDOX model. Oncotarget 2016; 7:12783-90; PMID:26859573
- Momiyama M, Zhao M, Kimura H, Tran B, Chishima T, Bouvet M, Endo I, Hoffman RM. Inhibition and eradication of human glioma with tumor-targeting Salmonella typhimurium in an orthotopic nude-mouse model. Cell Cycle 2012; 11:628-32; PMID:22274398; https://doi.org/10.4161/cc.11.3.19116
- Hiroshima Y, Zhao M, Zhang Y, Zhang N, Maawy A, Murakami T, Mii S, Uehara F, Yamamoto M, Miwa S, et al. Tumor-targeting Salmonella typhimurium A1-R arrests a chemo-resistant patient soft-tissue sarcoma in nude mice. PLoS One 2015; 10:e0134324; PMID:26237416; https://doi.org/10.1371/journal.pone.0134324
- Kiyuna T, Murakami T, Tome Y, Kawaguchi K, Igarashi K, Zhang Y, Zhao M, Li Y, Bouvet M, Kanaya F, et al. High efficacy of tumor-targeting Salmonella typhimurium A1-R on a doxorubicin- and dactolisib-resistant follicular dendritic-cell sarcoma in a patient-derived orthotopic xenograft PDOX nude mouse model. Oncotarget 2016; 7:33046-54; PMID:27105519
- Murakami T, Singh AS, Kiyuna T, Dry SM, Li Y, James AW, Igarashi K, Kawaguchi K, DeLong JC, Zhang Y, et al. Effective molecular targeting of CDK4/6 and IGF-1R in a rare FUS-ERG fusion CDKN2A-deletion doxorubicin-resistant Ewing's sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget 2016; 7:47556-64; PMID:27286459
- Kiyuna T, Murakami T, Tome Y, Igarashi K, Kawaguchi K, Russell T, Eckhardt MA, Crompton J, Singh A, Bernthal N, et al. Labeling the stroma of a patient-derived orthotopic xenograft (PDOX) mouse models of undifferentiated pleomorphic soft-tissue sarcoma with red fluorescent protein for rpaid non-invasive drug screening. J Cell Biochem 2017; 118:361-65; PMID:27357060; https://doi.org/10.1002/jcb.25643
- Yamamoto M, Zhao M, Hiroshima Y, Zhang Y, Shurell E, Eilber FC, Bouvet M, Noda M, Hoffman RM. Efficacy of tumor-targeting Salmonella typhimurium A1-R on a melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. PLoS One 2016; 11:e0160882; PMID:27500926; https://doi.org/10.1371/journal.pone.0160882
- Kawaguchi K, Murakami T, Chmielowski B, Igarashi K, Kiyuna T, Unno M, Nelson SD, Russell TA, Dry SM, Li Y, et al.. Vemurafenib-resistant BRAF-V600E mutated melanoma is regressed by MEK targeting drug trametinib, but not cobimetinib in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget 2016; 7:71737-43; PMID:27690220
- Kawaguchi K, Igarashi K, Murakami T, Chmiewloski B, Kiyuna T, Zhao M, Zhang Y, Singh A, Unno M, Nelson SD, et al. Tumor-targeting Salmonella typhimurium A1-R combined with Temozolomide regresses malignant melanoma with a BRAF-V600 mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget 2016; 7:85929-36; PMID:27835903
- Blagosklonny MV. Matching targets for selective cancer therapy. Drug Discov Today 2003; 8:1104-07; PMID:14678733; https://doi.org/10.1016/S1359-6446(03)02806-X
- Blagosklonny MV. Teratogens as anti-cancer drugs. Cell Cycle 2005; 4:1518-21; PMID:16258270; https://doi.org/10.4161/cc.4.11.2208
- Blagosklonny MV. Treatment with inhibitors of caspases, that are substrates of drug ransporters, selectively permits chemotherapy-induced apoptosis in multidrug-resistant cells but protects normal cells. Leukemia 2001; 15:936-41; PMID:11417480; https://doi.org/10.1038/sj.leu.2402127
- Blagosklonny MV. Target for cancer therapy: proliferating cells or stem cells. Leukemia 2006; 20:385-91; PMID:16357832; https://doi.org/10.1038/sj.leu.2404075
- Apontes P, Leontieva OV, Demidenko ZN, Li F, Blagosklonny MV. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget 2011; 2:222-33; PMID:21447859; https://doi.org/10.18632/oncotarget.248
- Blagosklonny MV. Tissue-selective therapy of cancer. Br J Cancer 2003; 89:1147-51; PMID:14520435; https://doi.org/10.1038/sj.bjc.6601256
- Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci USA 1992; 89:5645-49; PMID:1608975; https://doi.org/10.1073/pnas.89.12.5645
- Hiroshima Y, Maawy A, Zhang Y, Murakami T, Momiyama M, Mori R, Matsuyama R, Katz MH, Fleming JB, Chishima T, et al. Metastatic recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) nude mouse model is inhibited by neoadjuvant chemotherapy in combination with fluorescence-guided surgery with an anti-CA 19–9-conjugated fluorophore. Plos One 2014; 9:e114310; PMID:25463150; https://doi.org/10.1371/journal.pone.0114310
- Hiroshima Y, Maawy AA, Katz MH, Fleming JB, Bouvet M, Endo I, Hoffman RM. Selective efficacy of zoledronic acid on metastasis in a patient-derived orthotopic xenograph (PDOX) nude-mouse model of human pancreatic cancer. J Surg Oncol 2015; 111:311-5; PMID:25394368; https://doi.org/10.1002/jso.23816
- Fu X, Le P, Hoffman RM. A metastatic-orthotopic transplant nude-mouse model of human patient breast cancer. Anticancer Res 1993; 13:901-04; PMID:8352558
- Fu X, Hoffman RM. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res 1993; 13:283-6; PMID:8517640
- Wang X, Fu X, Hoffman RM. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int J Cancer 1992; 51:992-5; PMID:1639545; https://doi.org/10.1002/ijc.2910510621
- Hiroshima Y, Zhang Y, Zhang M, Maawy A, Mii S, Yamamoto M, Uehara F, Miwa S, Yano S, Murakami T, et al. Establishment of a patient-derived orthotopic xenograph (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLOS ONE 2015; 10:e0117417; PMID:25689852; https://doi.org/10.1371/journal.pone.0117417
- Fu X, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci USA 1991; 88:9345-9; PMID:1924398; https://doi.org/10.1073/pnas.88.20.9345
- Metildi CA, Kaushal S, Luiken GA, Talamini MA, Hoffman RM, Bouvet M. Fluorescently-labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Surg Oncol 2014; 109:451-8; PMID:24249594; https://doi.org/10.1002/jso.23507
- Hiroshima Y, Maawy A, Metildi CA, Zhang Y, Uehara F, Miwa S, Yano S, Sato S, Murakami T, Momiyama M, et al. Successful fluorescence-guided surgery on human colon cancer patient-derived orthotopic xenograft mouse models using a fluorophore-conjugated anti-CEA antibody and a portable imaging system. J Laparoendosc Adv Surg Tech A 2014; 24:241-47; PMID:24494971; https://doi.org/10.1089/lap.2013.0418
- Furukawa T, Kubota T, Watanabe M, Kitajima M, Fu X, Hoffman RM. Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: correlation of metastatic sites in mouse and individual patient donors. Int J Cancer 1993; 53:608-12; PMID:8436434; https://doi.org/10.1002/ijc.2910530414
- Hiroshima Y, Zhang Y, Zhang N, Uehara F, Maawy A, Murakami T, Mii S, Yamamoto M, Miwa S, Yano S, et al. Patient-derived orthotopic xenograft (PDOX) nude mouse model of soft-tissue sarcoma more closely mimics the patient behavior in contrast to the subcutaneous ectopic model. Anticancer Res 2015; 35:697-701; PMID:25667448
- Yamauchi K, Yang M, Jiang P, Xu M, Yamamoto N, Tsuchiya H, Tomita K, Moossa AR, Bouvet M, Hoffman RM. Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res 2006; 66:4208-14; PMID:16618743; https://doi.org/10.1158/0008-5472.CAN-05-3927