Fine-scale spatial and temporal control of translation control can have more rapid and subtle effects on the microenvironment of the cell than transcriptional regulation. Mammalian oocytes are a relevant model to study the spatial control of translation during meiotic maturation. Fully-grown mammalian oocytes are transcriptionally quiescent and stored maternal RNAs are used for the completion of meiosis and early embryo development. It was shown 3 decades ago that protein synthesis is not necessary for nuclear envelope breakdown (NEBD) during the resumption of meiosis but active protein synthesis is required for the correct formation of the meiotic spindle and progression to metaphase II.Citation1 Therefore, mammalian oocytes represent a good system to address translation control in relation to the cell cycle in general and with regard to spindle formation in particular. In this volume of the Cell Cycle the study by Jansova et al. provides new insight into the role of the eukaryotic initiation factor 4E-Binding Protein 1 (4E-BP1) in meiotic spindle formation in the mouse oocyte.Citation2
Phosphorylation of 4E-BP1 at sites that control its binding to eIF4E, and consequently its ability to inhibit translation, has been shown to be dynamic and finely regulated in space in mitotic and meiotic spindle.Citation3,4 In particular, it has been proposed that the localization of 4E-BP1 isoforms that have been phosphorylated at these sites at the spindle pole or on the spindle represents a novel mechanism to localize protein synthesis from mRNAs that are localized at the spindle. The study by Jansova et al. goes further by demonstrating that 4E-BP1-phosphorylation status influences whether a well-structured meiotic spindle is formed. Microinjection of mRNA encoding for a dominant negative 4E-BP1 mutant (replacement of key serine/threonine residues by non-phosphorylatable alanine residues) affected translation activity and induced aberrant spindle formation. Their data demonstrate that 4E-BP1-phosphorylation is essential to ensure correct meiotic progression.
Figure 1. CDK1 phosphorylates and activates mTOR, which phosphorylates 4E-BP1 on different sites (after (2)). Depending on the phosphorylated sites, 4E-BP is localized in the vicinity of the chromosomes and at the spindle poles (brown dashed arrows) or is distributed along the whole spindle (red dashed arrows).
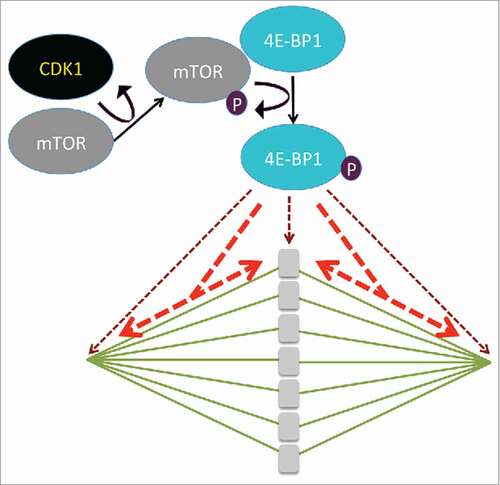
The identification of the kinases that are involved in the phosphorylation of 4E-BP1, represents a major challenge. Kubelka's group previously showed that chromosomal translational hotspots are controlled by the activity of the mTOR-signaling pathway during the first meiotic division in maturing mouse oocytes.Citation5 The current study adds new information by investigating the role of CDK1 in the phosphorylation of 4E-BP1 in mouse oocytes.Citation2 Using specific kinase inhibitors, they showed that CDK1 and mTOR kinases are the main positive regulators of 4E-BP1 phosphorylation following NEBD (). The Western blot data shown by Jansova et al. indicate that CDK1 influences the activity of mTOR in mouse oocytes suggesting that CDK1 acts indirectly on 4E-BP1 phosphorylation via mTOR activation. Interestingly, both CDK1 and mTOR co-localize with a specific phosphorylation isoform of 4E-BP1 on the spindle at the onset of meiotic resumption. The fact that CDK1 is a positive regulator of 4E-BP1-phosphorylation during mouse oocyte maturation will add fuel to the complicated debate about the link between translational activity and the cell cycle. Indeed, many discrepancies that have been reported when observing translation mechanisms linked with the cell cycle might be related to direct effect of the different pharmacological agents used to synchronize cells rather than cell cycle status itself.Citation6 Therefore, oocyte meiotic maturation, where the synchronism of cell divisions occurs physiologically, represents a pertinent model for the analysis of the control of the phosphorylation of 4E-BP1 by CDK1. The work by Jansova et al., which highlights the role of CDK1 in 4E-BP1 phosphorylation via the unexpected phosphorylation and activation of mTOR, adds complexity to the signaling pathway acting upstream of 4E-BP1 and may stimulate further research in this area.
In view of these studies, it now appears that fine control of translation activity within the spindle can play an important role in maintaining genomic stability. However, many points remain to be clarified. For example, how is phosphorylation of specific sites of 4E-BP1 controlled spatially and what is the consequence for translation activity of each modification? Which mRNAs are actively translated in this spatial and temporal context? The integration of knowledge about 4E-BP1-phosphorylation at different scales (molecular / structuralCitation7 and intracellular compartmentsCitation2-6) opens up a promising field of investigation for systemic approaches that focus on spatio-temporal control of mRNA translation. Several characteristics of the mammalian oocyte meiotic maturation system including its size and round shape, synchronism and asymmetry of meiotic divisions and links between meiotic divisions and translation, make it an interesting future model in this context.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Hashimoto N, Kishimoto T. Regulation of meiotic metaphase by a cytoplasmic maturation-promoting factor during mouse oocyte maturation. Dev Biol 1988; 126(2):242-52; PMID:3350209; http://dx.doi.org/10.1016/0012-1606(88)90135-2
- Jansova D, Koncicka M, Tetkova A, Cerna R, Malik R, del Llano E, et al. Regulation of 4E-BP1 activity in the mammalian oocyte. Cell Cycle 2017; In press; PMID:28272965; http://dx.doi.org/10.1080/15384101.2017.1295178.
- Romasko EJ, Amarnath D, Midic U, Latham KE. Association of maternal mRNA and phosphorylated EIF4EBP1 variants with the spindle in mouse oocytes: localized translational control supporting female meiosis in mammals. Genetics 2013; 195(2):349-58; PMID:23852387; http://dx.doi.org/10.1534/genetics.113.154005
- Shang ZF, Yu L, Li B, Tu WZ, Wang Y, Liu XD, Guan H, Huang B, Rang WQ, Zhou PK. 4E-BP1 participates in maintaining spindle integrity and genomic stability via interacting with PLK1. Cell Cycle 2012; 11(18):3463-71; PMID:22918237; http://dx.doi.org/10.4161/cc.21770
- Susor A, Jansova D, Cerna R, Danylevska A, Anger M, Toralova T, Malik R, Supolikova J, Cook MS, Oh JS, et al. Temporal and spatial regulation of translation in the mammalian oocyte via the mTOR-eIF4F pathway. Nat Commun 2015; 6:6078; PMID:25629602; http://dx.doi.org/10.1038/ncomms7078
- Coldwell MJ, Cowan JL, Vlasak M, Mead A, Willett M, Perry LS, Morley SJ. Phosphorylation of eIF4GII and 4E-BP1 in response to nocodazole treatment: a reappraisal of translation initiation during mitosis. Cell Cycle 2013; 12(23):3615-28; PMID:24091728; http://dx.doi.org/10.4161/cc.26588
- Bah A, Vernon RM, Siddiqui Z, Krzeminski M, Muhandiram R, Zhao C, Sonenberg N, Kay LE, Forman-Kay JD. Folding of an intrinsically disordered protein by phosphorylation as a regulatory switch. Nature 2015; 519:106-9; PMID:25533957; http://dx.doi.org/10.1038/nature13999
