Osteosarcoma (OS) is the most common malignant tumor in bone affecting children and adolescents. Since majority of the patients manifest micrometastases at the time of diagnosis, chemotherapy is the first line of treatment.Citation1 The 5-year survival rate for localized disease remains at 60–70% owing to advancement of neoadjuvant chemotherapy and surgery; however, there has been no significant improvement in the treatment of metastatic OS for the past 30 years with the survival rate remaining below 20%.Citation2 A deeper understanding of the mechanisms underlying malignant progression of OS is required for discovering better therapeutic approaches to target metastatic OS.
One of the hallmarks of malignant properties of cancer cells is their ability to survive in serum- and anchorage-independent conditions and form spheres, since cancer cells must overcome proliferation arrest and cell death induced by serum deprivation as well as loss of anchorage (anoikis).Citation3 In our recent study, we performed screening with a whole-genome human lentiviral shRNA library and identified an uncharacterized protein, transmembrane and immunoglobulin domain containing 3 (TMIGD3), as a factor whose downregulation significantly increased sphere-forming potential of OS cells.Citation4 There are 2 isoforms of TMIGD3, i1 and i3, that share a common C-terminal region with an immunoglobulin (Ig)-like fold (). However, the functions of TMIGD3 i1 and i3 remain largely unknown. Moreover, TMIGD3 i1 shares its N-terminal region (first 117 amino acids in exon 1) with adenosine A3 receptor (A3AR, ). A3AR, a well characterized Gi protein-associated G protein-coupled receptor (GPCR), is a member of the family of adenosine receptors including A1AR and A2AR, and is implicated in suppressing inflammation and cancer.Citation5 Activation of A3AR by adenosine or its agonists results in inhibition of adenylyl cyclase and reduction in cyclic AMP (cAMP) production, leading to suppression of multiple signaling pathways, including the Wnt-β-catenin, MAPK (mitogen-activated protein kinase), and NF-κB ().Citation6,7
Figure 1. Structures of cDNAs encoding TMIGD3/A3AR isoforms and their functions. A3AR/TMIGD3 isoforms locating on chromosome 1p13.2 have overlapping and non-overlapping function on cAMP-PKA-PKB/Akt-NF-κB axis and OS progression. A1, A2, and T1-T6 denote exons. ATG denotes start codon, while stop denotes stop codon. N.D.: not determined.
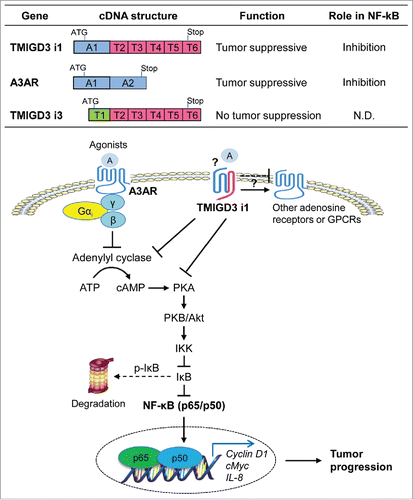
To understand the roles of TMIGD3 on OS progression, we query for the functional similarities between A3AR and TMIGD3, although neither TMIGD3 i1 nor i3 has a typical GPCR structure. Downregulation of TMIGD3 or A3AR by multiple shRNAs significantly increases malignant properties of different OS cell lines, including proliferation, migration, and sphere formation, as well as tumor progression and metastasis in nude mice.Citation4 Overexpression of A3AR and TMIGD3 i1, but not TMIGD3 i3, inhibits these malignant properties. Interestingly, similar to A3AR, TMIGD3 i1 suppresses activities of protein kinase A (PKA), PKB (also known as Akt), and NF-κB with minimal impacts on β-catenin and Erk1/2 activities, indicating that TMIGD3 i1 regulates overlapping signaling pathways with A3AR (). However, TMIGD3 i1 overexpression only partially rescues increase in sphere formation and cAMP production caused by A3AR knockdown. Moreover, unbiased luciferase-based pathway analyses reveal the presence of signaling pathways regulated by TMIGD3 distinct from A3AR. Thus, TMIGD3 i1 suppresses malignant properties of OS via both overlapping and non-overlapping pathways with A3AR.
Protein expression of TMIGD3 in OS tissues is significantly lower than that in normal bone and lungs, similar to A3AR.Citation4 Since transcription of both A3AR and TMIGD3 i1 is driven by the same promoter due to the common exon 1, expression of these proteins may be co-regulated. Consistently, high metastatic OS cell lines (KHOS/NP and MG63) tend to express lower levels of A3AR and TMIGD3 with lower IκB levels (indicating higher NF-κB activity), as compared with those in low-metastatic cell lines (U2OS and Saos-2).Citation4 It should be noted that shRNAs for TMIGD3 and an antibody for TMIGD3 used in our study could not discriminate between the 2 isoforms of i1 and i3. Hence, it would be crucial for identifying and using specific shRNAs and antibodies that can distinguish these isoforms in the future.
The existence of 2 potential tumor suppressors having similar functions in the same chromosome locus (1p13.2) in humans is quite intriguing. According to the Ensembl genome browser (http://uswest.ensembl.org/index.html), orthologous genes for TMIGD3 i1 are present in the majority of primates, as well as in horse, armadillo, pika, and lesser hedgehog tenrecs, while exceptionally gibbon possesses only gene orthologous for TMIGD3 i3 but not TMIGD3 i1. However, these species do not possess gene orthologous for A3AR. Many other vertebrates have orthologous genes for A3AR, but not TMIGD3 i1. Thus, Human is the only species which has both genes for A3AR and TMIGD3 i1. It would be interesting to understand the reasoning behind the natural selection of TMIGD3 i1 vs A3AR during evolution and why humans have evolved to possess both genes.
There are several questions still left unanswered, including how TMIGD3 i1 suppresses cAMP production, if TMIGD3 i1 could function as a GPCR, if adenosine could serve as a TMIGD3 i1 ligand, and whether TMIGD3 i1 inhibits progression of other types of cancer or suppresses immune-inflammatory disease as A3AR does. Meanwhile, it is also important to understand how TMIGD3 i1 regulates non-overlapping signaling pathways with A3AR and whether these pathways are involved in TMIGD3 i1-mediated inhibition of OS progression. Furthermore, understanding the mechanism behind reduced expression of A3AR and TMIGD3 i1 and identifying a strategy that restores the expression of these proteins would help discover a new therapy to reduce malignant properties of OS.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol 2015; 33:3029-35; PMID:26304877; http://dx.doi.org/10.1200/JCO.2014.59.4895
- Morrow JJ, Khanna C. Osteosarcoma genetics and epigenetics: emerging biology and candidate therapies. Crit Rev Oncog 2015; 20:173-97; PMID:26349415
- Adhikari AS, Agarwal N, Iwakuma T. Metastatic potential of tumor-initiating cells in solid tumors. Front Biosci 2011; 16:1927-38; PMID:21196274
- Iyer SV, Ranjan A, Elias HK, Parrales A, Sasaki H, Roy BC, Umar S, Tawfik OW, Iwakuma T. Genome-wide RNAi screening identifies TMIGD3 isoform1 as a suppressor of NF-kappaB and osteosarcoma progression. Nat Commun 2016; 7:13561; PMID:27886186; http://dx.doi.org/10.1038/ncomms13561
- Borea PA, Varani K, Vincenzi F, Baraldi PG, Tabrizi MA, Merighi S, Gessi S. The A3 adenosine receptor: history and perspectives. Pharmacol Rev 2015; 67:74-102; PMID:25387804
- Schulte G, Fredholm BB. Signaling pathway from the human adenosine A(3) receptor expressed in Chinese hamster ovary cells to the extracellular signal-regulated kinase 1/2. Mol Pharmacol 2002; 62:1137-46; PMID:12391277
- Fishman P, Bar-Yehuda S, Ohana G, Barer F, Ochaion A, Erlanger A, Madi L. An agonist to the A3 adenosine receptor inhibits colon carcinoma growth in mice via modulation of GSK-3 beta and NF-kappa B. Oncogene 2004; 23:2465-71; PMID:14691449; http://dx.doi.org/10.1038/sj.onc.1207355
