ABSTRACT
Centrioles are assembled during S phase and segregated into 2 daughter cells at the end of mitosis. The initiation of centriole assembly is regulated by polo-like kinase 4 (PLK4), the major serine/threonine kinase in centrioles. Despite its importance in centriole duplication, only a few substrates have been identified, and the detailed mechanism of PLK4 has not been fully elucidated. CP110 is a coiled-coil protein that plays roles in centriolar length control and ciliogenesis in mammals. Here, we revealed that PLK4 specifically phosphorylates CP110 at the S98 position. The phospho-resistant CP110 mutant inhibited centriole assembly, whereas the phospho-mimetic CP110 mutant induced centriole assembly, even in PLK4-limited conditions. This finding implies that PLK4 phosphorylation of CP110 is an essential step for centriole assembly. The phospho-mimetic form of CP110 augmented the centrosomal SAS6 level. Based on these results, we propose that the phosphorylated CP110 may be involved in the stabilization of cartwheel SAS6 during centriole assembly.
Introduction
The centrosome is a major microtubule organizing center and functions as a spindle pole during mitosis.Citation1 The centrosome consists of a centriole pair and the surrounding protein matrix, called pericentriolar material. A new centriole is assembled only once per cell cycle. Deregulation of centriole assembly may cause centrosome amplification, leading to aneuploidy, a hallmark of cancer cells.Citation2
PLK4 is one of the key molecules for the centriole assembly process.Citation3,Citation4 In fact, centriole assembly is blocked in the absence of PLK4 activity, whereas multiple centrioles are assembled in PLK4-overexpressing cells.Citation3,Citation4 A few centrosomal proteins have been identified as substrates of PLK4.Citation5-Citation7 STIL/Ana2 is the best-known substrate of PLK4 for centriole assembly.Citation8-Citation12 Phosphorylated STIL induces the recruitment of SAS6, a major cartwheel component, at the procentriole assembly site.Citation8-Citation12 For faithful centriole duplication, PLK4 activity should be tightly regulated throughout the cell cycle. SCF/Slimb E3 ubiquitin ligase is responsible for PLK4 degradation and prevents the enzyme overactivation.Citation13-Citation16 It was recently reported that KAT2A/B-mediated acetylation also limits hyperactivation of PLK4.Citation17 Of interest, STIL also functions as an activator of PLK4 at the centriole assembly site. The self-inhibitory structure of PLK4 is relieved by its physical interaction with STIL.Citation9,Citation11,Citation18
CP110 is a coiled-coil protein that is typically detected at the distal tip of centrioles. CP110 interacts with several centrosomal proteins and plays roles in centriolar length control and ciliogenesis, serving as a capping protein of centrioles.Citation19,Citation20 KIF24, a kinesin-13 family member with microtubule depolymerizing activity, is associated with CP110 and regulates centriole length.Citation21 CP110 depletion in Drosophila results in centriole length elongation that is overcome by co-depletion of Klp10A, another kinesin-13 family member.Citation22 The removal of CP110 from the mother centriole is essential for the initiation of ciliogenesis.Citation20 CEP97 interacts with CP110 for suppression of ciliogenesis,Citation23 whereas centrin-2 mediates the removal of CP110 from the distal end of the mother centriole, favoring cilia formation.Citation24
The role of CP110 is not limited to centriolar length control and ciliogenesis. In fact, CP110 is essential for centriole assembly. Its depletion blocked a rosette-like structure of procentrioles in PLK4-overexpressing cells.Citation3 CP110 was also identified as a substrate of CDK2/cyclin E for centriole duplication.Citation25 Of note, CP110 cellular levels must be tightly regulated during the cell cycle. Multiple regulatory mechanisms have been identified for CP110 expression. Cellular CP110 levels are significantly reduced during the G2/M phase due to ubiquitin-dependent degradation.Citation26 USP33, a deubiquitinating enzyme, antagonizes the ubiquitination of CP110 mainly in S phase when it is needed for centriole duplication.Citation27 The cellular CP110 level is carefully controlled for proper centriole assembly.Citation3,Citation25
In this study, we assessed how CP110 participates in the initial steps of procentriole assembly. Although CP110 is recruited early to the centriole assembly site, its significance in the centriole assembly process has not been clearly elucidated. Here, we identified CP110 as a novel substrate of PLK4. Furthermore, we confirmed that specific phosphorylation of CP110 is critical for centriole assembly.
Results
CP110 is phosphorylated by PLK4 in vitro and in vivo
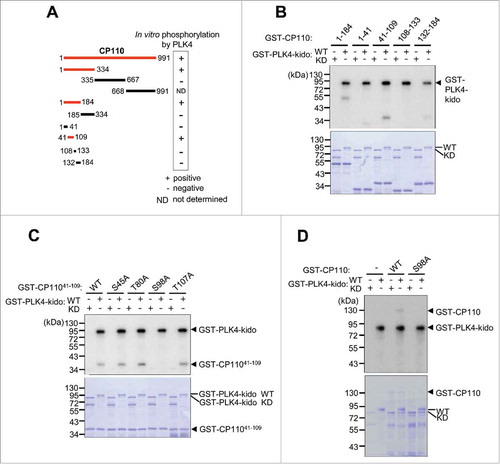
We performed in vitro kinase assays of PLK4 with CP110 as a potential substrate and found that the full-length CP110 fused to GST was phosphorylated by PLK4 (). After a series of in vitro kinase assays with the truncated and point mutants of GST-CP110, we limited the 41–109 fragment for major phosphorylation sites of PLK4 in vitro (). In vitro kinase assays with the alanine substitution mutants of GST-CP11041–109 at the presumptive phosphorylation sites pinpointed the serine residue at the 98th position of CP110 (S98CP110) as a PLK4 phosphorylation site (-). In fact, the GST-CP110S98A mutant protein was not phosphorylated by PLK4 in vitro (). Therefore, we concluded that the 98th serine residue of CP110 is a candidate phosphorylation site. Additional phosphorylation sites for PLK4 may be present given that the autoradiogram signals in the 1–41 and 132–184 fragments were weak but obvious (). Nonetheless, we began focusing on the biological significance of PLK4 phosphorylation of CP110 at S98. This phosphorylation site is conserved among human, monkey, mouse and chicken but not Xenopus and Takifugu (Fig. S1). In Drosophila, there is no N-terminal counterpart of human CP110.
Figure 1. CP110 is phosphorylated by PLK4 in vitro. (A) Summary of the in vitro kinase assays of PLK4. The red lines indicate the full-length or truncated CP110 proteins that were phosphorylated in vitro by PLK4. (B) In vitro kinase assays were performed with truncated fragments of GST-CP110 spanning the 1–184 residues. The wild type (WT) or kinase dead (KD) forms of GST-PLK4-kido (kinase domain) were used as enzymes. (C) In vitro kinase assays were performed with the GST-CP11041–109 proteins with alanine substitution mutations at the presumptive phosphorylation sites. (D) The full-length GST-CP110 (WT) and GST-CP110S98A (S98A) fusion proteins were finally tested for in vitro phosphorylation by GST-PLK4-kido. Protein levels were determined by Coomassie blue staining.
To test whether the S98 of CP110 is an actual phosphorylation site of PLK4, we generated a phospho-antibody specific to pS98CP110. The specificity of the phospho-antibody was examined by dot blot, coimmunostaining and immunoblot analyses ( and S2). The phospho-antibody specifically immunostained centrioles similar to the centrin-2 antibody (). Furthermore, the centrosomal signals of both CP110 and pS98CP110 were significantly reduced in CP110-depleted cells (). The centrosomal signal of pS98CP110 was reduced in PLK4-depleted cells, suggesting its dependency on PLK4 activity (). Immunoblot analyses revealed that the pS98CP110-specific band was detected in the CP110 immunoprecipitates of the control cells but not in PLK4-depleted cells ( and S3). These results suggest that S98 of CP110 is specifically phosphorylated by PLK4 in vivo.
Figure 2. CP110 is phosphorylated by PLK4 in vivo. (A) CP110-depleted U2OS cells were treated with thymidine for 20 h and coimmunostained with the centrin-2 antibody (green) and CP110 or pS98CP110 antibodies (red). The centrosomal intensities of CP110 and pS98CP110 were determined in greater than 30 centrosomes per experimental groups in 3 independent experiments. (B) The PLK4-depleted HeLa cells were arrested at M phase with the STLC treatment of 3 h and coimmunostained with antibodies specific to centrin-2 (green) and pS98CP110 (red). The centrosomal intensities of pS98CP110 were determined in greater than 30 centrosomes per experimental groups in 3 independent experiments. (C) The PLK4-depleted HeLa cells were lysed and subjected to immunoprecipitation with anti-CP110 serum. Immunoblot analysis was performed with the CP110 and pS98CP110 antibodies. Preimmune serum (PI) was used as a control. A larger version of the blot images is presented in Fig. S3. (D) The M phase-arrested HeLa cells were synchronously released to G1 phase, cultured for up to 12 h and coimmunostained with γ-tubulin antibody (red) along with the CP110 or pS98CP110 antibodies (green). Greater than 10 centrosomes per experimental groups were assessed. (E) HeLa cells were coimmunostained with γ-tubulin antibody (red) along with CP110 or pS98CP110 antibodies (green). Mitotic stages were determined with DAPI (blue). Greater than 10 centrosomes per experimental groups were determined. Values are means and standard deviations. The statistical significance was determined by paired t-tests. *, P<0.05.
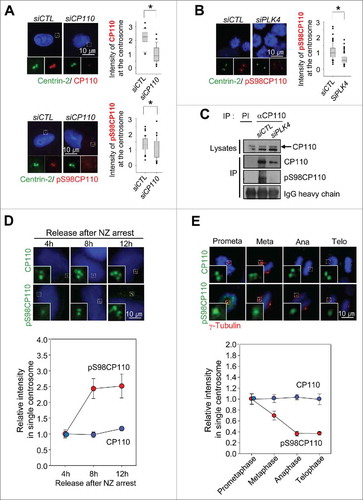
We determined the centrosomal CP110 and pS98CP110 levels during the cell cycle. HeLa cells were arrested at the M phase with nocodazole and released into fresh medium. At the indicated time points after the release, the cells were coimmunostained with antibodies specific to CP110 and pS98CP110. The results demonstrated that the centrosomal signals of pS98CP110 significantly increased 8 h after the nocodazole release when centrioles duplicate, whereas CP110 signals were relatively constant (). We also determined the intensities of centrosomal pS98CP110 during mitosis. The results demonstrated that CP110 phosphorylation started to decrease from metaphase and was reduced to basal levels in anaphase, whereas the centrosomal CP110 levels remained constant (). We also observed that the specific phosphorylation of CP110 was exclusively detected in the centrioles of cycling cells but not resting cells with cilia (Fig. S4). Taken together, CP110 proteins are phosphorylated during S phase when new procentrioles assemble, and they are dephosphorylated during mitosis at the centrosome. Our results are consistent with the previous study in which PLK4 expression increases in S phase through G2 phase and peaks at M phase.Citation28,Citation29
The phospho-resistant mutant of CP110 inhibits centriole duplication
We examined the importance of PLK4 phosphorylation of CP110. First, U2OS cells were transiently transfected with Flag-GFP, Flag-CP110, Flag-CP110S98A or Flag-CP110S98E. All the ectopic Flag-CP110 proteins were localized at the centrosomes (). Ectopic expression of Flag-CP110S98A significantly reduced the number of cells with duplicated centrioles, whereas ectopic expression of Flag-CP110 and Flag-CP110S98E did not affect centriole duplication (). These data reveal that the phospho-resistant CP110S98A mutant had a dominant-negative effect on centriole assembly.
Figure 3. Inhibitory effects of the phospho-resistant Flag-CP110S98A mutant on centriole duplication. (A) U2OS cells were transiently transfected with pFlag-GFP, pFlag-CP110WT, pFlag-CP110S98A or pFlag-CP110S98E. The transfected cells were treated with thymidine for 20 h. Expression of ectopic CP110 proteins was confirmed by immunoblot analysis. The asterisk indicates a nonspecific band. The cells were coimmunostained with antibodies specific to Flag (green) and centrin-2 (red). DNA was stained with DAPI (blue). The number of centrioles per cell was counted based on centrin-2 signals. Greater than 200 cells per experimental group were analyzed in 4 independent experiments. (B) Endogenous CP110 was depleted in U2OS cells. After siRNA transfection, the cells were rescued with siRNA-resistant constructs of pFlag-CP110WT, pFlag-CP110S98A or pFlag-CP110S98E. The cells were then treated with hydroxyurea (HU) for 72 h. Expression of endogenous CP110 and ectopic CP110 proteins was confirmed by immunoblot analysis. The cells were coimmunostained with antibodies specific to Flag (green) and γ-tubulin (red). DNA was stained with DAPI (blue). The centrosome number was assessed based on γ-tubulin signals. Greater than 300 cells per experimental group were analyzed in 3 independent experiments. Values are means and standard deviations. *, P <0.05.
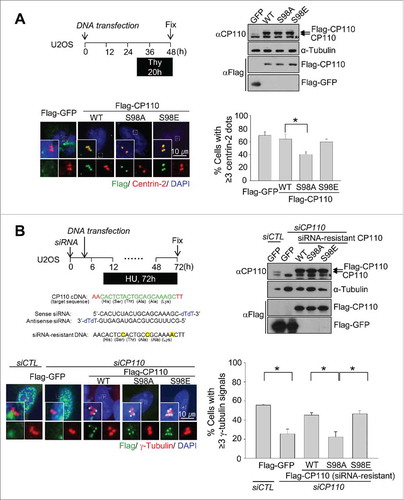
The importance of CP110 phosphorylation in centriole duplication was also examined in S phase-arrested U2OS cells. Endogenous CP110 was depleted with siRNA transfections, and the siRNA-resistant constructs of CP110 (Flag-CP110, Flag-CP110S98A and Flag-CP110S98E) were subsequently introduced into the cells. The cells were then treated with hydroxyurea for 72 h to induce centrosome overduplication. As expected, centrosome overduplication was inhibited with depletion of CP110 and rescued with ectopic expression of Flag-CP110 and Flag-CP110S98E (). However, centrosome overduplication was not rescued by ectopic expression of Flag-CP110S98A (). These results suggest that PLK4 phosphorylation of CP110 at S98 is required for centrosome duplication.
The phospho-mimetic mutant of CP110 supports centriole duplication in PLK4-reduced conditions
We examined whether the phospho-mimetic CP110S98E mutant could rescue centriole assembly in PLK4-depleted cells. We generated Tet-on U2OS stable cell lines in which the expression of Flag-GFP, Flag-GFP-CP110, Flag-GFP-CP110S98A or Flag-GFP-CP110S98E was instantly induced with doxycycline (). Endogenous PLK4 was simultaneously depleted with siRNA transfections. As expected, the number of cells with duplicated centrioles was significantly reduced upon PLK4 depletion ( and S5). This phenotype was effectively rescued by the ectopic expression of Flag-GFP-CP110S98E but not Flag-GFP-CP110 or Flag-GFP-CP110S98A ( and S5).
Figure 4. Induction of centriole duplication with the phospho-mimetic CP110S98E mutant in PLK4-reduced conditions. (A) Expression of ectopic Flag-GFP, Flag-GFP-CP110WT, Flag-GFP-CP110S98A or Flag-GFP-CP110S98E was induced in Tet-on U2OS stable cell lines. Endogenous PLK4 was depleted with siRNA transfections. Immunoblot analysis confirmed the expression of ectopic CP110 proteins. The cells were treated with hydroxyurea for 26 h and coimmunostained with antibodies specific to Flag (green) and centrin-2 (red). DNA was stained with DAPI (blue). The number of centrioles per cell was counted. Greater than 200 cells per experimental group were analyzed in 3 independent experiments. (B) Flag-GFP-, Flag-GFP-CP110WT- and Flag-GFP-CP110S98E-expressing U2OS cells were treated with 100 nM centrinone, a PLK4 inhibitor, for 36 h and coimmunostained with antibodies specific to Flag (green) and centrin-2 (red). DNA was stained with DAPI (blue). Greater than 180 cells per experimental group were analyzed in 3 independent experiments. Values are means and standard deviations. *, P<0.05.
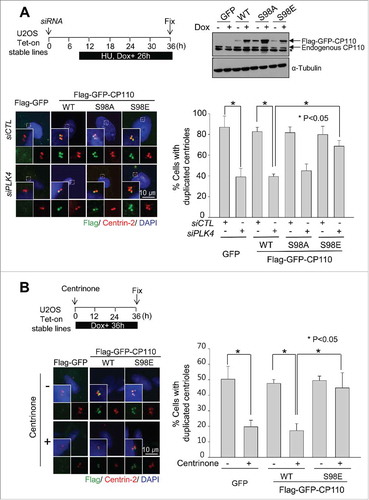
Next, we used centrinone, a specific inhibitor of PLK4, to confirm the importance of PLK4 phosphorylation of CP110 in centriole assembly.Citation30 Centrinone effectively inhibited centriole duplication in Tet-on U2OS stable cell lines expressing Flag-GFP-CP110 (). However, centriole assembly in Flag-GFP-CP110S98E-expressing cells was not inhibited by centrinone treatment (). These results support the notion that specific phosphorylation of CP110 is required for centriole assembly even with a reduced PLK4 activity.
Augmentation of centrosomal SAS6 with the phospho-mimetic mutant of CP110
Centrosomal recruitment of SAS6, a cartwheel protein, is one of the earliest events in centriole assembly.Citation31,Citation32 Centriolar localization of SAS6 depends on the PLK4 phosphorylation of STIL during centriole assembly.Citation10,Citation12 We examined whether the PLK4 phosphorylation of CP110 mediates the centriolar localization of SAS6. Ectopic Flag-CP110 proteins were expressed in Tet-on U2OS stable cell lines, and the centrosomal levels of SAS6 were determined. The results showed that ectopic expression of Flag-CP110S98E augmented centrosomal SAS6 levels, whereas Flag-CP110 or Flag-CP110S98A did not (). Centrosomal SAS6 levels were reduced with centrinone treatment but remained significantly increased in the Flag-CP110S98E-expressing cells (). However, centrosomal STIL levels in the Flag-CP110S98E-expressing cells slightly increased but did not change as much as noted in centrosomal SAS6 (Fig. S6). These results support the notion that PLK4 phosphorylation of CP110 mediates the centrosomal localization of SAS6 but not STIL.
Figure 5. Augmentation of the centrosomal SAS6 with the phospho-mimetic CP110S98E mutant. (A) Flag-GFP-, Flag-GFP-CP110-, Flag-GFP-CP110S98A- and Flag-GFP-CP110S98E-expressing U2OS cells were treated with 100 nM centrinone for 48 h and coimmunostained with antibodies specific to SAS6 (red) and Flag (green). DNA was stained with DAPI (blue). Relative intensities of the centrosomal SAS6 were measured from 100 centrosomes in 2 independent experiments and analyzed with box-and whisker plots. The bottom and top of each box mark the 25th and 75th percentiles, respectively. The whiskers are drawn down to the 5th percentile and up to the 95th. Points that lie outside the 5th and 95th percentiles are plotted as symbols. *P < 0.05, paired t-test. (B) U2OS stable cell lines were transfected with siSAS6. Forty-eight hours later, the cells were subjected to immunoblot analysis to confirm depletion of SAS6. The cells were also immunostained with the centrin-2 antibody to determine the number of centrioles. Greater than 100 cells were counted per experimental group in 3 independent experiments. Values are means and standard deviations.
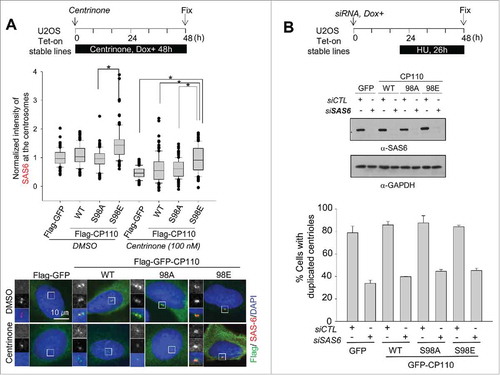
We previously demonstrated that the phospho-mimetic mutant of CP110 (Flag-CP110S98E) supported centriole assembly in PLK4-limited conditions (). Therefore, we next examined whether the same phospho-mimetic mutant could also induce centriole assembly in SAS6-depleted conditions. Depletion of SAS6 inhibited centriole duplication as expected (). However, the wild type and the phospho-mimetic mutant of CP110 did not induce centriole duplication in SAS6-depleted cells (). These results suggest that the phospho-mimetic CP110 may overcome the limited availability of PLK4 for centriole assembly but may not bypass the requirement of SAS6.
Discussion
In this study, we revealed that CP110 is a novel substrate of PLK4. Our finding is consistent with the recent report in which CP110 interacts with PLK4 in a yeast two-hybrid screen.Citation33 The phospho-resistant mutant of CP110 inhibited centriole assembly, whereas the phospho-mimetic mutant induced centriole assembly even in PLK4-limited conditions. These observations imply that PLK4 phosphorylation of CP110 is an essential step for centriole assembly.
The best-known function of CP110 may be the regulation of centriolar microtubules. KIF24A, a microtubule depolymerizer, interacts with CP110 at the distal end of centrioles and controls microtubule growth to maintain proper centriolar length. Therefore, it is surprising that CP110 is a substrate of PLK4 that should be concentrated at the centriole assembly sites near the proximal ends not the distal ends of centrioles. However, the sub-centriolar localization of CP110 at the proximal end has been previously suggested. First, a fraction of ectopic CP110 is detected at the proximal end of mother centrioles in Drosophila cells.Citation34 Second, USP33, a deubiquitinating enzyme of CP110, is located near the proximal side, suggesting that a subpopulation of CP110 is located at the proximal ends of centrioles.Citation27 These previous observations imply that CP110 may function at the centriole assembly site in an early phase.
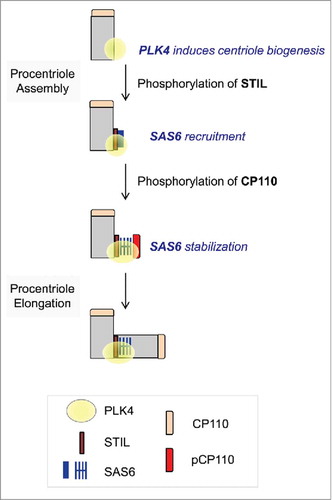
Although it is speculative to predict the role of CP110 in centriole assembly, we propose that the phosphorylated CP110 may be involved in the stable incorporation of centriolar components, such as SAS6, during centriole assembly (). The proposal is based on the observation that ectopic expression of phospho-mimetic CP110 augmented the centrosomal SAS6 levels (). Under the tested conditions, the inhibition of PLK4 by centrinone reduced centrosomal SAS6 levels. Interestingly, the phospho-mimetic CP110 mutant augmented centrosomal SAS6 levels not only in normal conditions but also in PLK4-limited conditions (). Therefore, centriole assembly may proceed even with low PLK4 activity if SAS6 is recruited and efficiently stabilized with the help of the phospho-mimetic CP110. We assume that both STIL and CP110 should be phosphorylated by PLK4 for the successful loading of SAS6 onto the newly forming centrioles at an early phase of centriole assembly (). The phospho-mimetic CP110 may compensate for reduced STIL function for SAS6 recruitment in PLK4-limited conditions. In support of this hypothesis, ectopic expression of the phospho-resistant CP110 inhibited centriole duplication ().
Figure 6. Model. Centriole assembly initiates at the proximal end of mother centriole where PLK4 is concentrated. STIL phosphorylation by PLK4 mediates SAS6 recruitment.Citation28,Citation29 CP110 is also phosphorylated by PLK4. This event may stabilize SAS6 during the initial procentriole assembly.
Our proposal is also based on the fact that CP110 interacts with multiple centrosomal proteins, some of which are essential for centriole length control.Citation20 For the initiation of centriole duplication, protein-protein interactions were proposed to be critical.Citation33 PLK4 phosphorylation might control CP110 interactions with specific centrosomal proteins for centriole assembly. We have not identified a centrosome protein whose interaction with CP110 is controlled by specific phosphorylation to date. PLK4 phosphorylation did not affect the known CP110 interactions with CEP97 (data not shown).
The specific functions of PLK4 in centriole assembly have begun to be elucidated. PLK4 phosphorylates STIL/Ana2, a SAS5 homolog, and facilitates its interaction with SAS6.Citation9,Citation10,Citation12 As a result, a centriolar cartwheel is formed next to the mother centriole as the earliest event of procentriole assembly.Citation10,Citation12 PLK4 phosphorylates CEP135 to properly position the essential centriole component CEP152/Asterless.Citation33 Other centriolar proteins are also identified as substrates of PLK4. GCP6, a component of γTuRC, is phosphorylated by PLK4 and involved in PLK4-induced centriole overduplication.Citation5 SCF-FBXW5, an E3-ubiquitin ligase that regulates SAS6 levels in a cell cycle stage-specific manner, is another substrate of PLK4.Citation35 PCM-1, a centriolar satellite protein, was recently added to the list of PLK4 substrates, thus implying PLK4 function in organizing the centriolar satellites.Citation36 These results suggest that PLK4 phosphorylation of multiple target proteins is required for assembling centrioles.
Our results demonstrate that CP110 is a novel substrate of PLK4 and CP110 phosphorylation is necessary and required for efficient centriole assembly. On the contrary, CP110-deleted Drosophila cells normally assemble centrioles during development.Citation34 This discrepancy might stem from the differences in experimental systems of mammalian cultured cells and Drosophila embryonic cells. It is possible that centriolar components in Drosophila embryos may be sufficiently abundant such that CP110 may not be critically necessary. CP110 in Drosophila embryos plays a supporting role in centriole assembly along with other assembly factors. To clarify CP110 function in centriole assembly, we may further focus on identifying centrosomal proteins whose interaction with CP110 is regulated by PLK4 phosphorylation.
Materials and methods
Cell culture and plasmids
HeLa cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) at 37°C and 5% CO2. U2OS cells were grown in McCoy's 5A supplemented with 10% FBS. Cells were synchronized to enrich M phase cells with nocodazole (100 ng/ml) or to enrich S phase cells with 2 mM thymidine or 2 mM hydroxyurea. U2OS cells were chosen for the induction of centriole overduplication in S-phase arrested cells. HeLa cells were selected due to the efficiency of the synchronization.
The human CP110 cDNA clone was subcloned into the 3 × Flag vector (Sigma) and TRE vector (Clontech). The phospho-resistant and mimetic mutants of CP110 were generated by site-directed mutagenesis. The sequence of siRNA-resistant CP110 is as follows: Target CP110 sequence, AA CAC(His) TCT(Ser) ACT(Thr) GCA(Ala) GCA(Ala) AAG(Lys) CTT; siRNA-resistant construct, AA CAC(His) TCC(Ser) ACT(Thr) GCC(Ala) GCA(Ala) AAA(Lys) CTT. Plasmids were transfected with FuGENE HD (Roche).
Double stable cell lines were developed by stably transfecting Tet-on U2OS cells with plasmids containing Flag-GFP, Flag-GFP-CP110, Flag-GFP-CP110S98A or Flag-GFP-CP110S98E under the control of the Tet-response element. Stable cell lines with ectopic CP110 proteins were selected with 400 μg/ml hygromycin, and the expression of the recombinant CP110 proteins was induced with doxycycline treatment (0.2 μg/ml). For the inhibition of PLK4 activity, U2OS stable cell lines were treated with 100 nM centrinone, a specific PLK4 inhibitor (a kind gift from K. Oegema, U. of California, San Diego).
siRNAs and antibodies
The siRNAs were purchased (ST Pharm, Korea) and the sequences are as follows: siCP110 (CAC UCU ACU GCA GCA AAG C-dTdT), siCTL (scrambled sequence for control) (GCA AUC GAA GCU CGG CUA C-dTdT), siPLK4 (CUA UCU UGG AGC UUU AUA A-dTdT), siSAS6 (GCA CGU UAA UCA GCU ACA A-dTdT) and siSTIL (GCU CCA AAC AGU UUC UGC U-dTdT). The siRNAs were transfected into cells using RNAi MAX reagents (Invitrogen).
The CP110 antibody was raised against GST-CP1101–334.Citation37 The centrin-2 and CEP135 polyclonal antibodies were raised against GST-centrin-21–172 and GST-CEP135295–1141, respectively.Citation38 The pS98CP110 phospho-antibody was raised in a rabbit against the phospho-peptides corresponding to the residues CRKAPNApSDFDOW and affinity-purified with phospho- and non-phospho-peptides. The peptides were purchased from AbClon Co., Ltd, Korea. For the generation of anti-rabbit polyclonal antibodies, a rabbit was purchased, housed and handled according to the guidelines of the ethic committee at Seoul National University. The antibodies against γ-tubulin (sc-7396, Santa Cruz Biotechnology, Inc.), centrin-2 (04–1624, Millipore), Flag (F3165, Sigma), SAS-6 (sc-81431, sc-98506 Santa Cruz Biotechnology, Inc.), STIL (ab89314, Abcam) and acetylated tubulin (T6793, Sigma) were purchased. Additional antibodies used for immunoblotting include the antibodies against α-tubulin (T6199, Sigma) and GAPDH (AM4300, Ambion). HRP-conjugated secondary antibodies (A9044, Sigma and AP132P, Millipore) were used at 1:10,000 dilutions.
Immunoprecipitation and Immunoblotting
Immunoprecipitation and immunoblot analysis were performed as described previously.Citation39 In brief, the cells were washed with PBS and lysed with the ELB buffer (50 mM HEPES pH 7.0, 250 mM NaCl, 5 mM EDTA, 0.1% NP40, 1 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 10 mM NaF, 50 mM β–glycerophosphate and 10% glycerol). The lysates were placed on ice for 40 min and centrifuged for 20 min at 4˚C. The soluble fraction was then incubated with the anti-CP110 serum for 4–6 h. After incubation with antibodies, the cell lysate was mixed with protein A sepharose beads (Amersham Pharmacia) for 90 min at 4˚C and washed thrice with the lysis buffer. The immunoprecipitate was eluted with the SDS sample buffer (50 mM Tris-HCl, pH 6.8, 100 mM dithiothreitol, 2% SDS, 0.1% bromophenol blue and 10% glycerol).
For immunoblotting, the cells were lysed with the SDS sample buffer, subjected to SDS-PAGE, and transferred onto a nitrocellulose membrane. The membranes were blocked in 5% skim milk in TBST (20 mM Tris-Cl pH 8.0, 150 mM NaCl and 0.1% Tween 20) for 1 h, incubated with primary antibodies overnight at 4˚C, and washed thrice with TBST. After incubation with HRP-conjugated secondary antibodies for 40 min, the membrane was washed with TBST and visualized with ECL solutions.
Immunofluorescence microscopy
For indirect immunocytochemistry, HeLa and U2OS cells were grown on 12-mm coverslips and fixed with cold methanol for 10 min. In some cases, cells were extracted with 0.1% PBST (0.1% Triton X-100 in PBS) before fixation. The fixed cells were then blocked with PBS containing 3% BSA and 0.5% Triton X-100 for 15 min and incubated with the primary antibodies for 1–2 h at room temperature. The cells were washed with 0.1% PBST and subsequently incubated with the secondary antibodies conjugated with Alexa Fluor-488, 594 or 647. DNA was counterstained with DAPI solution. The samples were mounted in ProLong Gold anti-fade reagent (Invitrogen) and observed with a fluorescence microscope (Olympus IX51) equipped with a CCD camera (Qicam fast 1394, Qimaging). The images were processed using ImagePro 5.0 (Media Cybernetics, Inc.).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KCCY_A_1325555_Supplemental.pdf
Download PDF (464.8 KB)Acknowledgment
We are grateful to Dr. Karen Oegema (U. of California, San Diego) who provided centrinone.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry (NRF-2014R1A4A1005259 and 2016R1A2B4009418).
References
- Luders J, Stearns T. Microtubule-organizing centres: A re-evaluation. Nat Rev Mol Cell Biol 2007; 8(2):161-7; PMID:17245416; https://doi.org/10.1038/nrm2100
- Gordon DJ, Resio B, Pellman D. Causes and consequences of aneuploidy in cancer. Nat Rev Genet 2012; 13(3):189-203; PMID:22269907
- Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA. Plk4-induced centriole biogenesis in human cells. Dev Cell 2007; 13(2):190-202; PMID:17681131; https://doi.org/10.1016/j.devcel.2007.07.002
- Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. The polo kinase plk4 functions in centriole duplication. Nat Cell Biol 2005; 7(11):1140-6; PMID:16244668; https://doi.org/10.1038/ncb1320
- Bahtz R, Seidler J, Arnold M, Haselmann-Weiss U, Antony C, Lehmann WD, Hoffmann I. Gcp6 is a substrate of plk4 and required for centriole duplication. J Cell Sci 2012; 125(Pt 2):486-96; PMID:22302995; https://doi.org/10.1242/jcs.093930
- Hatch EM, Kulukian A, Holland AJ, Cleveland DW, Stearns T. Cep152 interacts with plk4 and is required for centriole duplication. J Cell Biol 2010; 191(4):721-9; PMID:21059850; https://doi.org/10.1083/jcb.201006049
- Hori A, Barnouin K, Snijders AP, Toda T. A non-canonical function of plk4 in centriolar satellite integrity and ciliogenesis through pcm1 phosphorylation. EMBO Rep 2016; 17(3):326-37; PMID:26755742; https://doi.org/10.15252/embr.201541432
- Kratz AS, Barenz F, Richter KT, Hoffmann I. Plk4-dependent phosphorylation of stil is required for centriole duplication. Biol Open 2015; 4(3):370-7; PMID:25701666; https://doi.org/10.1242/bio.201411023
- Moyer TC, Clutario KM, Lambrus BG, Daggubati V, Holland AJ. Binding of stil to plk4 activates kinase activity to promote centriole assembly. J Cell Biol 2015; 209(6):863-78; PMID:26101219; https://doi.org/10.1083/jcb.201502088
- Ohta M, Ashikawa T, Nozaki Y, Kozuka-Hata H, Goto H, Inagaki M, Oyama M, Kitagawa D. Direct interaction of plk4 with stil ensures formation of a single procentriole per parental centriole. Nat Commun 2014; 5:5267; PMID:25342035; https://doi.org/10.1038/ncomms6267
- Arquint C, Gabryjonczyk AM, Imseng S, Bohm R, Sauer E, Hiller S, Nigg EA, Maier T. Stil binding to polo-box 3 of plk4 regulates centriole duplication. Elife 2015; 4:e07888.
- Dzhindzhev NS, Tzolovsky G, Lipinszki Z, Schneider S, Lattao R, Fu J, Debski J, Dadlez M, Glover DM. Plk4 phosphorylates ana2 to trigger sas6 recruitment and procentriole formation. Curr Biol 2014; 24(21):2526-32; PMID:25264260; https://doi.org/10.1016/j.cub.2014.08.061
- Cunha-Ferreira I, Rodrigues-Martins A, Bento I, Riparbelli M, Zhang W, Laue E, Callaini G, Glover DM, Bettencourt-Dias M. The scf/slimb ubiquitin ligase limits centrosome amplification through degradation of sak/plk4. Curr Biol 2009; 19(1):43-9; PMID:19084407; https://doi.org/10.1016/j.cub.2008.11.037
- Guderian G, Westendorf J, Uldschmid A, Nigg EA. Plk4 trans-autophosphorylation regulates centriole number by controlling betatrcp-mediated degradation. J Cell Sci 2010; 123(Pt 13):2163-9; PMID:20516151; https://doi.org/10.1242/jcs.068502
- Rogers GC, Rusan NM, Roberts DM, Peifer M, Rogers SL. The scf slimb ubiquitin ligase regulates plk4/sak levels to block centriole reduplication. J Cell Biol 2009; 184(2):225-39; PMID:19171756; https://doi.org/10.1083/jcb.200808049
- Holland AJ, Lan W, Niessen S, Hoover H, Cleveland DW. Polo-like kinase 4 kinase activity limits centrosome overduplication by autoregulating its own stability. J Cell Biol 2010; 188(2):191-8; PMID:20100909; https://doi.org/10.1083/jcb.200911102
- Fournier M, Orpinell M, Grauffel C, Scheer E, Garnier JM, Ye T, Chavant V, Joint M, Esashi F, Dejaegere A et al. Kat2a/kat2b-targeted acetylome reveals a role for plk4 acetylation in preventing centrosome amplification. Nat Commun 2016; 7:13227; PMID:27796307; https://doi.org/10.1038/ncomms13227
- Klebba JE, Buster DW, McLamarrah TA, Rusan NM, Rogers GC. Autoinhibition and relief mechanism for polo-like kinase 4. Proc Natl Acad Sci U S A 2015; 112(7):E657-66; PMID:25646492; https://doi.org/10.1073/pnas.1417967112
- Schmidt TI, Kleylein-Sohn J, Westendorf J, Le Clech M, Lavoie SB, Stierhof YD, Nigg EA. Control of centriole length by cpap and cp110. Curr Biol 2009; 19(12):1005-11; PMID:19481458; https://doi.org/10.1016/j.cub.2009.05.016
- Tsang WY, Dynlacht BD. Cp110 and its network of partners coordinately regulate cilia assembly. Cilia 2013; 2(1):9; PMID:24053599
- Kobayashi T, Tsang WY, Li J, Lane W, Dynlacht BD. Centriolar kinesin kif24 interacts with cp110 to remodel microtubules and regulate ciliogenesis. Cell 2011; 145(6):914-25; PMID:21620453; https://doi.org/10.1016/j.cell.2011.04.028
- Delgehyr N, Rangone H, Fu J, Mao G, Tom B, Riparbelli MG, Callaini G, Glover DM. Klp10a, a microtubule-depolymerizing kinesin-13, cooperates with cp110 to control drosophila centriole length. Curr Biol 2012; 22(6):502-9; PMID:22365849; https://doi.org/10.1016/j.cub.2012.01.046
- Spektor A, Tsang WY, Khoo D, Dynlacht BD. Cep97 and cp110 suppress a cilia assembly program. Cell 2007; 130(4):678-90; PMID:17719545; https://doi.org/10.1016/j.cell.2007.06.027
- Prosser SL, Morrison CG. Centrin2 regulates cp110 removal in primary cilium formation. J Cell Biol 2015; 208(6):693-701; PMID:25753040; https://doi.org/10.1083/jcb.201411070
- Chen Z, Indjeian VB, McManus M, Wang L, Dynlacht BD. Cp110, a cell cycle-dependent cdk substrate, regulates centrosome duplication in human cells. Dev Cell 2002; 3(3):339-50; PMID:12361598; https://doi.org/10.1016/S1534-5807(02)00258-7
- D'Angiolella V, Donato V, Vijayakumar S, Saraf A, Florens L, Washburn MP, Dynlacht B, Pagano M. Scf(cyclin f) controls centrosome homeostasis and mitotic fidelity through cp110 degradation. Nature 2010; 466(7302):138-42; PMID:20596027; https://doi.org/10.1038/nature09140
- Li J, D'Angiolella V, Seeley ES, Kim S, Kobayashi T, Fu W, Campos EI, Pagano M, Dynlacht BD. Usp33 regulates centrosome biogenesis via deubiquitination of the centriolar protein cp110. Nature 2013; 495(7440):255-9; PMID:23486064; https://doi.org/10.1038/nature11941
- Fode C, Binkert C, Dennis JW. Constitutive expression of murine sak-a suppresses cell growth and induces multinucleation. Mol Cell Biol 1996; 16(9):4665-72; PMID:8756623; https://doi.org/10.1128/MCB.16.9.4665
- Swallow CJ, Ko MA, Siddiqui NU, Hudson JW, Dennis JW. Sak/plk4 and mitotic fidelity. Oncogene 2005; 24(2):306-12; PMID:15640847; https://doi.org/10.1038/sj.onc.1208275
- Wong YL, Anzola JV, Davis RL, Yoon M, Motamedi A, Kroll A, Seo CP, Hsia JE, Kim SK, Mitchell JW et al. Cell biology. Reversible centriole depletion with an inhibitor of polo-like kinase 4. Science (New York, NY) 2015; 348(6239):1155-60; https://doi.org/10.1126/science.aaa5111
- Delattre M, Canard C, Gonczy P. Sequential protein recruitment in c. Elegans centriole formation. Curr Biol 2006; 16(18):1844-9
- Pelletier L, O'Toole E, Schwager A, Hyman AA, Muller-Reichert T. Centriole assembly in caenorhabditis elegans. Nature 2006; 444(7119):619-23; PMID:17136092; https://doi.org/10.1038/nature05318
- Galletta BJ, Fagerstrom CJ, Schoborg TA, McLamarrah TA, Ryniawec JM, Buster DW, Slep KC, Rogers GC, Rusan NM. A centrosome interactome provides insight into organelle assembly and reveals a non-duplication role for plk4. Nat Commun 2016; 7:12476; PMID:27558293; https://doi.org/10.1038/ncomms12476
- Franz A, Roque H, Saurya S, Dobbelaere J, Raff JW. Cp110 exhibits novel regulatory activities during centriole assembly in drosophila. J Cell Biol 2013; 203(5):785-99; PMID:24297749; https://doi.org/10.1083/jcb.201305109
- Puklowski A, Homsi Y, Keller D, May M, Chauhan S, Kossatz U, Grunwald V, Kubicka S, Pich A, Manns MP et al. The scf-fbxw5 e3-ubiquitin ligase is regulated by plk4 and targets hssas-6 to control centrosome duplication. Nat Cell Biol 2011; 13(8):1004-9; PMID:21725316; https://doi.org/10.1038/ncb2282
- Hori A, Barnouin K, Snijders AP, Toda T. A non-canonical function of plk4 in centriolar satellite integrity and ciliogenesis through pcm1 phosphorylation. EMBO Rep 2016; 17(3):326-37; PMID:26755742
- Chang J, Cizmecioglu O, Hoffmann I, Rhee K. Plk2 phosphorylation is critical for cpap function in procentriole formation during the centrosome cycle. EMBO J 2010; 29(14):2395-406; PMID:20531387; https://doi.org/10.1038/emboj.2010.118
- Kim K, Lee S, Chang J, Rhee K. A novel function of cep135 as a platform protein of c-nap1 for its centriolar localization. Exp Cell Res 2008; 314(20):3692-700; PMID:18851962; https://doi.org/10.1016/j.yexcr.2008.09.016
- Kim J, Lee K, Rhee K. Plk1 regulation of pcnt cleavage ensures fidelity of centriole separation during mitotic exit. Nat Commun 2015; 6:10076; PMID:26647647; https://doi.org/10.1038/ncomms10076
