The circadian clock system represents an anticipatory mechanism, playing an essential role in the temporal coordination of metabolism in the physiological context, and in etiology of metabolic disorders.Citation1 Cell-autonomous self-sustained circadian oscillators are functional in virtually all cells in the body. Such body-web of circadian clocks must be synchronized on a daily basis, with the light-dark cycle being the principle synchronizer or Zeitgeber (time-giver from German), which impacts on rhythmic systemic signals that are controlled by the master pacemaker in the suprachiasmatic nucleus (SCN) of the brain. Furthermore, oscillators operative in peripheral tissues and cells can be synchronized by a variety of signals, allowing their investigation in vitro.Citation2
The circadian system plays a key role in the regulation of body metabolism, including glucose homeostasis via coordination of hormone secretion by the endocrine pancreas on one hand, and glucose utilization by skeletal muscle and adipose tissue on the other.Citation1 A functional unit of the endocrine pancreas, the pancreatic islet of Langerhans, represents a 3-dimensional structure of distinct types of endocrine cells, tightly packed together in an organized and species-specific way. While intercellular oscillator coupling is indispensable for SCN neuron synchronization, peripheral clocks do not show coupling within organs.Citation3 In line with these findings, mouse and human intact pancreatic islets and dispersed islet cells exhibit pronounced circadian oscillations, suggesting that physical interaction between neighboring cells within the 3-dimensional islet architecture is not crucial for the cellular oscillator function.Citation4
Although fundamental aspects concerning the circadian control of transcriptional and functional regulation in whole pancreatic islets have recently been assessed,Citation5 our knowledge regarding individual islet cell molecular clocks and their inputs and outputs is still limited. The bi-hormonal hypothesis proposed by Unger and Orci back in 1975 emphasizes that the perturbation of glucose homeostasis in the context of diabetes mellitus is stemming from altering the balance between the glucose regulating counter hormones insulin and glucagon, rather than from the pathology of insulin alone.Citation6 Clearly, acute glucose sensing plays an essential role in the temporal separation of glucagon and insulin secretion by adjacent α- and β-cells. Our recent parallel study of the circadian clockwork in glucagon-producing α-cells along with insulin producing β-cells, separated from each other following pancreatic islets isolation based on cell specific fluorescent reporters, revealed additional plausible mechanism for such temporal coordination.Citation4 Strikingly, α- and β-cellular clocks exhibited distinct circadian properties, including a phase difference between α- and β-cell oscillators that was observed in vivo and in vitro, at population and at single-cell levels (,Citation4). Such phase coherence, which was kept upon islet cell synchronization in vitro with physiologically relevant stimuli such as adrenaline, is likely to be mediated by the distinct repertoire of receptors expressed on α- and β-cell surfaces, as it is the case for the adrenergic receptors. Moreover, basal secretion profiles of glucagon and insulin by mixed and separated α- and β-cell cultures revealed oscillatory profiles for both islet hormones, further suggesting that cell-autonomous islet oscillators are likely to contribute importantly to the orchestration of these temporal secretion patterns (,Citation4). Large-scale in vivo transcriptome analysis, conducted in separated α- and β-cells, revealed that a high number of key islet genes exhibit oscillatory profiles in either one or both islet cell types. Rhythmically expressed transcripts with similar or distinct characteristics in α- and β-cells comprised those encoding for glucose transporters, glucose metabolic enzymes, and regulators of granule trafficking and exocytosis, which may account for the cyclic pattern of islet hormone secretionCitation4 as has been previously suggested by whole-islet analysis.Citation5
Figure 1. Interplay between α- and β-cellular clocks plays a role in the temporal coordination of insulin and glucagon secretion. Circadian oscillators operative in α-cells tend to lag behind β-cellular clocks. This phase coherence between α- and β-cellular clocks may contribute to the phase-shift observed between insulin and glucagon secretion profiles.Citation4 Differential transcriptional regulation of α-and β-cell functional genes related to hormone granule assembly, trafficking and exocytosis, might represent a plausible link between the cellular oscillator and the phase of hormone secretion.
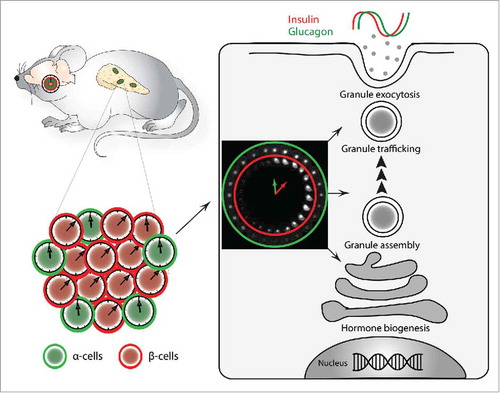
Collectively, these findings elucidate that beyond acute control mechanisms of insulin or glucagon secretion, driven by feeding-fasting conditions and reflected by fast changes in blood glucose levels, there is an additional mechanism governed by cell-autonomous islet clocks. This latter regulatory machinery is coordinating the secretion of insulin and glucagon in a daytime-dependent manner, that ensures anticipation of changes related to rest-activity and feeding-fasting cycles, resulting in the optimal adaptation of both key glucose regulating islet hormones to the organismal needs. Notably, circadian regulation of basal insulin secretion by human pancreatic islets has been recently demonstrated, pointing out that this mechanism might be kept across species.Citation7 Furthermore, the absolute levels and the circadian profile of insulin secretion showed a clear dependence on the presence of functional cell-autonomous islet clocks. In the context of a growing need for efficient treatments of metabolic disorders, future studies of α- and β-cellular clocks upon obesity and type 2 diabetes in humans hold promise for identifying new avenues for therapeutic approaches based on the modulation of cellular clocks.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Dr. Evgenia Andreoli for the graphic work, and Dr. Ursula Loizides-Mangold for the text proofreading.
References
- Gachon F, Loizides-Mangold U, Petrenko V, Dibner C. Glucose homeostasis: regulation by peripheral circadian clocks in rodents and humans. Endocrinology 2017; [Epub ahead of print]; PMID:28324069; https://doi.org/10.1210/en.2017-00218
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 1998; 93:929-37; PMID:9635423; https://doi.org/10.1016/S0092-8674(00)81199-X
- Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 2007; 129:605-16; PMID:17482552; https://doi.org/10.1016/j.cell.2007.02.047
- Petrenko V, Saini C, Giovannoni L, Gobet C, Sage D, Unser M, Heddad Masson M, Gu G, Bosco D, Gachon F, et al. Pancreatic alpha- and beta-cellular clocks have distinct molecular properties and impact on islet hormone secretion and gene expression. Genes Dev 2017; 31:383-98; https://doi.org/10.1101/gad.290379.116
- Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, Peek CB, Hong H, Huang W, Omura C, et al. Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 2015; 350:aac4250; PMID:26542580; https://doi.org/10.1126/science.aac4250
- Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1975; 1:14-6; PMID:46337; https://doi.org/10.1016/S0140-6736(75)92375-2
- Saini C, Petrenko V, Pulimeno P, Giovannoni L, Berney T, Hebrok M, Howald C, Dermitzakis ET, Dibner C. A functional circadian clock is required for proper insulin secretion by human pancreatic islet cells. Diabetes Obesity Metab 2016; 18:355-65; https://doi.org/10.1111/dom.12616
