Mitotic division depends on the phosphorylation of numerous proteins that orchestrate the structural rearrangements of the cell. These multiple phosphorylations are under the control of the Cdk1-Cyclin B complex/MPF (M-phase promoting factor). Recently, Cdk1-cyclin B counteracting phosphatase has been characterized as PP2A-B55.Citation1 Hence, the events controlling mitotic entry and exit is now known to be controlled by the balance of Cdk1-cyclin B kinase and PP2A-B55 phosphatase. The molecular mechanisms triggering PP2A-B55 activation/inhibition have been identified thanks to the extensive data on the Greatwall kinase (Gwl).Citation2 At G2-M transition Gwl is activated and triggers the phosphorylation of its substrates Arpp19 and ENSA. This modification occurs on a conserved consensus site yfdSgdy (S62/67 and 67/67 in human and Xenopus Arpp19 and ENSA substrates respectively). Once phosphorylated by Gwl, Arpp19/ENSA directly bind and inhibit PP2A-B55 triggering the stable phosphorylation of Cdk1-cyclin B substrates and mitotic entry. Upon mitotic exit, cyclin B-Cdk1 and Gwl kinases are inactivated, PP2A-B55 activity raises and mitotic substrates are dephosphorylated.Citation3,4
Besides the well-established role of Cdk1-cyclin B-dependent phosphorylation of Arpp19 in mitosis/meiosis, Arpp19 has been shown to be also phosphorylated by cAMP-dependent protein kinase PKA (S107 in humans and S109 in Xenopus), however much less is known about the functional role of this phosphorylation.
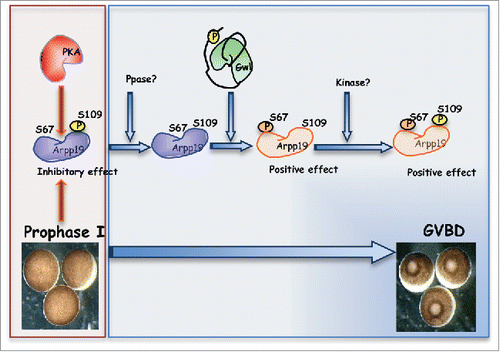
Within the last 3 years, Dupre, Haccard and Jessus have clearly established that the main target of PKA required for the maintenance of prophase I arrest in vertebrate oocytes is Arpp19.Citation5 Supporting this hypothesis, these authors showed that Arpp19 phosphorylation on S109 by PKA blocks meiotic resumption in Xenopus oocytes. Moreover, they demonstrate that upon meiotic re-entry, following PKA activity drop, Arpp19 becomes first dephosphorylated on its PKA site and subsequently re-phosphorylated again on this residue concomitantly with the phosphorylation on the Gwl site S67. Interestingly, since PKA activity is low throughout meiotic division, a distinct unknown kinase must be responsible of this phosphorylation. Once Arpp19 phosphorylated on S67 by Gwl, Cdk1-cyclin B is fully activated and meiotic progression takes place. Now, in the present study Dupre et al. uses “in vitro” phosphorylation and Xenopus oocyte microinjection of several Arpp19 mutant forms to investigate the functional interplay between S67 and S109 phosphorylations on Arpp19. Their “in vitro” results demonstrate that the pre-phosphorylation of Arpp19 on S109 by PKA does not affect the subsequent Gwl-dependent phosphorylation of this protein on S67. In addition, they showed that although when microinjected on prophase I oocytes the S109 thio-phosphorylated form of Arpp19 blocks progesterone-induced oocyte maturation, the thio-phosphorylated form of this protein on both S109 and S67 results in PP2A-B55 inhibition and meiotic progression indicating that phosphorylation on S67 is dominant with respect to S109.Citation6 Next, the authors performed similar experiments with S109A or S109D mutant thiophosphorylated in vitro by Gwl at S67. Microinjected into prophase oocytes, both proteins induced meiotic maturation. However, Cdk1 activation promoted by S109A-S67P-Arpp19 protein is less efficient than that by S109D-S67P-Arpp19 suggesting that once phosphorylated at S67, the phosphorylation on S109 residue conveys some positive effect. This point should be confirmed in future experiments.
Besides Gwl and PKA, Cdk1 also phosphorylates Arpp19 at serine 28 in Xenopus. Okumura et al. reported that the Cdk1 phosphorylation of Arpp19 is critical for meiotic division in starfish oocyte. This study therefore highlights the possibility that Cdk1-dependent phosphorylation of Arpp19 could be sufficient to promote meiotic maturation.Citation7
In the present study, Dupre et al. adressed this question by micro-injecting the Cdk1 inhibitor P21 into G2-arrested Xenopus oocytes. In this context, both thio-S67 and thio-S67-S109A-Arpp19 mutants efficiently bound PP2A-B55 in the absence of Cdk1 activity suggesting that Cdk1-dependent phosphorylation of Arpp19 would only play a minor effect on meiotic maturation. Accordingly, the microinjection of prophase I-arrested oocytes with either a phosphomimetic or a thio-phosphorylated form of Arpp19 on the Cdk1 phosphorylation site did induce meiotic resumption unless progesterone was added into the media. The authors concluded that phosphorylation on S28 is dispensable for Arpp19 ability to promote meiotic maturation.
The last question addressed by the authors concerns the physiologic role of Arpp19 rephosphorylation on S109 concomitantly with its S67 phosphorylation upon meiotic resumption observed in their previous studies. In this regard, they asked whether this rephosphorylation could impact on MPF auto-amplification loop. To answer this question, authors micro-injected into Xenopus oocytes a combination of thio-phosphorylated and double Arpp19 mutants: ThioS67-S109A Arpp19 and S67A-S109D Arpp19. Microinjection of the former directly activated the MPF auto-amplification loop whereas the latter inhibited meiosis resumption induced by progesterone. From these results they concluded that the phosphorylation of S109 does not counterbalance the ability of S67-phosphorylated Arpp19 to promote MPF auto-amplification loop through PP2A-B55 inactivation.
The importance of Arpp19 protein in meiosis/mitotic control is becoming clearer, but more research is needed to fully understand its regulations throughout meiotic maturation, from prophase I to metaphase II.
Figure 1. How phosphorylation controls Arpp19 functions? PKA phosphorylates S109 of Arpp19 at prophase I of meiosis. Hormonal stimulation promotes S109 dephosphorylation. The phosphatase responsible of this dephosphorylation remains to be characterized. Next, Gwl phosphorylates S67 of Arpp19 and an unknown kinase phosphorylates S109 of Arpp19 protein.Citation5
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Ligue Nationale Contre le Cancer (LNCC-Equipe Labellisée).
References
- Mochida S, Ikeo S, Gannon J, Hunt T. Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J 2009; 28:2777-85; PMID:19696736; https://doi.org/10.1038/emboj.2009.238
- Vigneron S, Brioudes E, Burgess A, Labbe JC, Lorca T, Castro A. Greatwall maintains mitosis through regulation of PP2A. EMBO J 2009; 28:2786-93; PMID:19680222; https://doi.org/10.1038/emboj.2009.228
- Gharbi-Ayachi A, Labbe JC, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 2010; 330:1673-7; PMID:21164014; https://doi.org/10.1126/science.1197048
- Mochida S, Maslen SL, Skehel M, Hunt T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 2010; 330:1670-3; PMID:21164013; https://doi.org/10.1126/science.1195689
- Dupré A, Daldello EM, Nairn AC, Jessus C, Haccard O. Phosphorylation of ARPP19 by protein kinase A prevents meiosis resumption in Xenopus oocytes. Nat Commun 2014; 5:3318; PMID:24525567; https://doi.org/10.1038/ncomms4318
- Dupre A, Haccard O, Jessus C. The Greatwall kinase is dominant over PKA in controlling the antagonistic function of ARPP19 in Xenopus oocytes. Cell Cycle 2017; 16(15):1440-1452; PMID:28722544; https://doi.org/10.1080/15384101
- Okumura E, Morita A, Wakai M, Mochida S, Hara M, Kishimoto T. Cyclin B-Cdk1 inhibits protein phosphatase PP2A-B55 via a Greatwall kinase-independent mechanism. J Cell Biol 2014; 204:881-9; PMID:24616226; https://doi.org/10.1083/jcb.201307160
