The mitotic spindle is a bipolar array of microtubules, which orchestrates the accurate segregation of chromosomes during cell division. To build this complex structure, microtubules need to be formed at the right time and location inside the cell. Besides originating from spindle poles or around chromosomes, microtubules were most recently found to originate from microtubules themselves, termed branching microtubule nucleation.Citation1 Because the branch angle is very shallow, microtubules amplify themselves with the same polarity, giving rise to dense structures composed of parallel microtubules, such as the spindle. Branching microtubule nucleation requires the universal microtubule nucleator gamma-tubulin ring complex (γTuRC), the protein complex augmin, and the protein TPX2.Citation1 The latter is released from inhibitory importins by the small GTPase Ran in the vicinity of chromosomes (, top),Citation2 and it has gathered substantial attention as a major driver of spindle assembly.
Figure 1. Top: RanGTP releases TPX2 from inhibitory importin α in the vicinity of chromosomes during mitosis. Bottom: Schematic of the TPX2 protein highlighting the location of key functional elements, as well as different truncated TPX2 constructs with their observed abilities to nucleate microtubules from purified tubulin in vitro, and to stimulate branching microtubule nucleation in Xenopus egg extract.
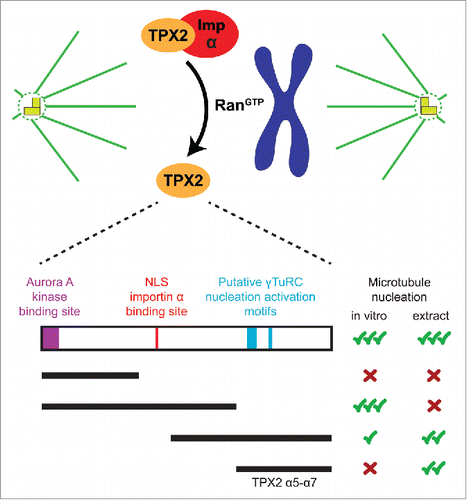
TPX2 was originally identified as a microtubule-associated protein that targets the kinesin Xklp2 to spindle poles. However, it quickly became clear that upon its release by Ran, TPX2 plays a critical role in generating the microtubules that make up the spindle.Citation2 In an effort to understand this function, a variety of interaction partners and properties have been described for TPX2. Its N-terminal half activates Aurora A kinase, which subsequently stimulates microtubule generation around chromatin by phosphorylating spindle proteins.Citation3 This region of TPX2 is also important to promote microtubule formation from purified tubulin in vitro.Citation4 On the other hand, the C-terminal half of TPX2 is sufficient to induce robust levels of microtubule nucleation in Xenopus egg extract (, bottom).Citation1,4 Finally, aside from its role in nucleation, TPX2 can directly affect microtubule plus-end dynamics in vitro.Citation5 Despite these important findings, and others not mentioned here, the exact molecular mechanism by which TPX2 stimulates microtubule nucleation in vivo was still not known.
To dissect TPX2s function, we established the domain organization of Xenopus laevis TPX2 using secondary structure analysis, based on which we defined the minimal TPX2 fragment (TPX2 α5-α7, amino acids 478–716) that stimulates branching microtubule nucleation in Xenopus egg extract (, bottom).Citation6 Imaging this cell-free extract with TIRF microscopy allowed us to visualize and quantify individual nucleation events over time, which is not possible in the cell, and is unique to our assay system. We confirmed that TPX2 α5-α7 does not retain the full-length's ability to intrinsically nucleate microtubules from purified tubulin, suggesting that this function of TPX2 is not essential to stimulate branching microtubule nucleation. This finding also implies that TPX2 requires or acts through another factor to perform its function. Supporting this idea, we found that TPX2 does not stimulate microtubule nucleation in egg extract depleted of the protein complex augmin,Citation6 which has been proposed to recruit γTuRC to pre-existing microtubules. This surprising result suggests that TPX2 only promotes microtubule nucleation from pre-existing microtubules, at least within the timescale in which branching microtubule nucleation is observed.
It has long been speculated that γTuRC must be activated to build the microtubule cytoskeleton, and key motifs in putative activator proteins have been identified.Citation7 Knowing that γTuRC is required for branching microtubule nucleation, we identified sequences within the minimal TPX2 α5-α7 fragment that share homology with known γTuRC nucleation activator motifs (, bottom). These sequences are necessary for TPX2s activity in Xenopus egg extract, and proteins with single-site mutations introduced in these motifs still bind to γTuRC, but no longer stimulate branching microtubule nucleation.Citation6 These findings suggest that TPX2 plays the γTuRC activator role within the branching microtubule nucleation pathway. However, our results do not rule out the possibility that the minimal TPX2 fragment could recruit another factor from the extract. Because TPX2 cannot activate microtubule nucleation in the absence of augmin, an intact complex between TPX2, augmin and γTuRC, and potentially other proteins, may be necessary to achieve this activation. After all, the spatial and functional regulation of these factors must be such that they act together in a single, or in sequential steps to eventually form a new microtubule from the side of a pre-existing one. To ultimately test the concrete mechanism of TPX2, it will be necessary to reconstitute this reaction from purified components in vitro in the future.
Finally, it will also be important to understand whether and how the other functions of TPX2 are used in branching microtubule nucleation. For example, while the N-terminal half of TPX2 alone cannot induce branching microtubule nucleation, full-length TPX2 generates branched microtubules more efficiently than the C-terminal half alone.Citation6 Additionally, the N-terminal half is important to facilitate microtubule nucleation from purified tubulin in vitro, and it contains the binding site for Aurora A kinase, which also plays a role in generating microtubules around chromatin in Xenopus egg extract (, bottom).Citation3,4 Therefore, we speculate that these 2 features of the N-terminal half, and potentially other properties, such as the ability to directly affect microtubule plus-end dynamics,Citation5 contribute to the increased efficiency of full-length TPX2 in promoting microtubule nucleation. Future research will elucidate the molecular mechanism of how TPX2 induces the formation of new microtubules from pre-existing ones together with augmin and γTuRC, and how this activity contributes to the spatiotemporal regulation of microtubule nucleation during spindle assembly.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Petry S, Groen AC, Ishihara K, Mitchison TJ, Vale RD. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell 2013; 152:768-77; PMID:23415226; https://doi.org/10.1016/j.cell.2012.12.044
- Gruss OJ, Carazo-Salas RE, Schatz CA, Guarguaglini G, Kast J, Wilm M, Le Bot N, Vernos I, Karsenti E, Mattaj IW. Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell 2001; 104:83-93; PMID:11163242; https://doi.org/10.1016/S0092-8674(01)00193-3
- Scrofani J, Sardon T, Meunier S, Vernos I. Microtubule nucleation in mitosis by a RanGTP-dependent protein complex. Curr Biol 2015; 25:131-40; PMID:25532896; https://doi.org/10.1016/j.cub.2014.11.025
- Brunet S, Sardon T, Zimmerman T, Wittmann T, Pepperkok R, Karsenti E, Vernos I. Characterization of the TPX2 domains involved in microtubule nucleation and spindle assembly in Xenopus egg extracts. Mol Biol Cell 2004; 15:5318-28; PMID:15385625; https://doi.org/10.1091/mbc.E04-05-0385
- Reid TA, Schuster BM, Mann BJ, Balchand SK, Plooster M, McClellan M, Coombes CE, Wadsworth P, Gardner MK. Suppression of microtubule assembly kinetics by the mitotic protein TPX2. J Cell Sci 2016; 129:1319-28; PMID:26869224; https://doi.org/10.1242/jcs.178806
- Alfaro-Aco R, Thawani A, Petry S. Structural analysis of the role of TPX2 in branching microtubule nucleation. J Cell Biol 2017; 216:983-97; PMID:28264915; https://doi.org/10.1083/jcb.201607060
- Lin TC, Neuner A, Schlosser YT, Scharf AN, Weber L, Schiebel E. Cell-cycle dependent phosphorylation of yeast pericentrin regulates gamma-TuSC-mediated microtubule nucleation. Elife 2014; 3:e02208; PMID:24842996; https://doi.org/10.7554/eLife.02208
