ABSTRACT
Separase cleaves cohesin to allow chromosome segregation. Separase also regulates cortical granule exocytosis and vesicle trafficking during cytokinesis, both of which involve RAB-11. We investigated whether separase regulates exocytosis through a proteolytic or non-proteolytic mechanism. In C. elegans, protease-dead separase (SEP-1PD::GFP) is dominant negative. Consistent with its role in cohesin cleavage, SEP-1PD::GFP causes chromosome segregation defects. As expected, partial depletion of cohesin rescues this defect, confirming that SEP-1PD::GFP acts through a substrate trapping mechanism. SEP-1PD::GFP causes cytokinetic defects that are synergistically exacerbated by depletion of the t-SNARE SYX-4. Furthermore, SEP-1PD::GFP delays furrow ingression, causes an accumulation of RAB-11 vesicles at the cleavage furrow site and delays the exocytosis of cortical granules during anaphase I. Depletion of syx-4 further enhanced RAB-11::mCherry and SEP-1PD::GFP plasma membrane accumulation during cytokinesis, while depletion of cohesin had no effect. In contrast, centriole disengagement appears normal in SEP-1PD::GFP embryos, indicating that chromosome segregation and vesicle trafficking are more sensitive to inhibition by the inactive protease. These findings suggest that separase cleaves an unknown substrate to promote the exocytosis of RAB-11 vesicles and paves the way for biochemical identification of substrates.
Introduction
Faithful cell division depends on coordinated regulation of chromosome segregation and cytokinesis. Chromosome segregation requires equal partitioning of sister chromatids that are duplicated and linked together by cohesin during mitotic S-phase.Citation1 At the onset of anaphase, the kleisin subunit of cohesin, SCC-1, is cleaved by the caspase-like cysteine protease separase, allowing sister chromatid separation.Citation2 Separase is a large protease with 2 sub-domains, the pseudo-protease domain (PPD) and active protease domain (APD) as well as an extended helical repeat region in the N-terminus.Citation3-7 The canonical role of separase is to cleave SCC-1, which allows chromosome segregation during mitotic and meiotic anaphase in all eukaryotic organisms studied to date.Citation8 The proteolytic function of separase is required for several other cell cycle events in anaphase. In budding yeast, separase cleaves the kinetochore and spindle associated protein Slk19, which stabilizes the anaphase spindle.Citation9,10 Additionally, separase cleaves the pericentriolar material proteins kendrin and pericentrin B to regulate centriole licensing in mammalian cells.Citation11,12 Interestingly, separase cleaves itself at multiple adjacent sites.Citation13-15 The auto-cleaved fragments still maintain catalytic activity, and self-cleavage plays important roles in controlling cell cycle progression, separase activity and chromosome segregation.Citation16,17 These proteolytic functions stress the importance of identifying the distinct roles of separase and its substrates in both meiosis and mitosis.
In addition to its roles as a protease, several non-proteolytic functions of separase have been identified. At anaphase onset, separase-dependent activation of the Cdc14 early anaphase release (FEAR) pathway initiates mitotic exit in budding yeast.Citation18 A protease dead separase mutant is still sufficient to initiate mitotic exit but cannot promote cohesin cleavage and spindle elongation.Citation19 Interestingly, Cdc14 has been shown to promote cytokinesis by regulating ER to bud neck trafficking of chitin synthase and directly dephosphorylating several bud neck targets.Citation20-24 Separase is also known to bind and inhibit CDK-1 in mammalian cells through an unstructured region between the catalytic and N-terminal domain.Citation6,25-27 Consistent with this, several studies have shown that expression of catalytically inactive separase can rescue multiple aspects of separase function.Citation26,28 In oocytes, expression of inactive separase can rescue polar body extrusion, a highly asymmetric form of cytokinesis, after knockdown of endogenous separase.Citation28 These earlier studies would suggest the hypothesis that protease dead separase might be capable of promoting the cytokinetic functions of separase. However, our unexpected observation that protease dead separase is dominant negative in C. elegans suggests that it interferes with endogenous separase function.Citation29 This provides a novel opportunity to investigate the cellular functions that are affected by protease dead separase.
Caenorhabditis elegans is a powerful model system for addressing fundamental cell cycle events. Oocytes mature and undergo fertilization every 25 minutes, then complete meiosis and initiate the mitotic cell divisions within an hour in utero, all of which can be imaged with relative ease.Citation30 In C. elegans, separase performs multiple functions during the oocyte-to-embryo transition in the first meiotic division and the mitotic metaphase-to-anaphase transition. Separase is essential for homologous chromosome disjunction through cleaving meiosis-specific kleisin subunit Rec8.Citation31 During anaphase I, separase cleaves the CENP-A related protein, CPAR-1, which may regulate the metaphase-anaphase transition in C. elegans.Citation32 Separase is involved in centriole disengagement during male spermatocyte meiosisCitation33 and regulates the separation and duplication of sperm-derived centrioles in embryos at the meiosis-mitosis transition.Citation34 During mitosis, separase cleaves the mitotic cohesin kleisin subunit SCC-1 to promote chromosome segregation.Citation31,35 Whether C. elegans separase has the same conserved non-proteolytic functions such as CDK-1 inhibition is unknown, as is whether other protease dead separase mutants are dominant negative in other systems.
Our previous studies have defined an essential function for separase in the regulation of vesicle exocytosis during anaphase. Separase inactivation causes eggshell defects and cytokinesis failures, both of which are due to defects in vesicle trafficking. During anaphase I, separase localizes to cortical granules and is required for their exocytosis, which is necessary for eggshell formation.Citation36 Simultaneously, separase localizes to the base of the polar body and is required for successful cytokinesis during polar body extrusion (PBE). RAB-11, a small GTPase that regulates trafficking at recycling endosomes and is essential for cytokinesis in several systems, is also found on cortical granules and the base of the polar body and is required for both events in anaphase I.Citation37 Further study indicated that separase is also required for cytokinesis during mitosis.Citation38 Interestingly, depletion of separase in C. elegans with RNAi enhanced the accumulation of RAB-11 positive vesicles at the ingressing furrow and midbody, suggesting a role of separase in exocytosis during cytokinesis.Citation38 Furthermore, the role of separase in exocytosis is independent of its function in chromosome segregation as a unique hypomorphic mutant that maps to the N-terminal domain promotes mostly normal chromosome segregation, while cortical granule exocytosis (CGE) and cytokinesis remain severely affected.Citation36,38 These studies demonstrate that CGE is under the control of the same cellular machinery that regulates membrane trafficking during polar body extrusion and mitotic cytokinesis. Separase has been also found in plant and mammalian systems to regulate membrane trafficking,Citation39,40 suggesting that separase may have a conserved function in regulating membrane trafficking.
There are many open questions about the exact mechanism of how separase regulates RAB-11 vesicle exocytosis. Previous studies in mouse oocytes suggest that separase has a non-proteolytic role in polar body extrusion, and thus possibly in vesicle trafficking.Citation28 However, we recently reported the unexpected observation that SEP-1PD::GFP is dominant negative in C. elegans.Citation29 Here, we investigated cellular phenotypes to understand what processes are impaired by SEP-1PD::GFP in C. elegans and whether vesicle trafficking is affected. We used high-resolution confocal microscopy to observe SEP-1PD::GFP phenotypes during meiosis I and mitotic cytokinesis. We show that SEP-1PD::GFP impairs both chromosome segregation and RAB-11 vesicle trafficking, but does not impact centriole disengagement. Depletion of the substrate, cohesin scc-1, substantially rescues chromosome bridging during anaphase in SEP-1PD::GFP embryos, consistent with the hypothesis that SEP-1PD::GFP prevents substrate cleavage. SEP-1PD::GFP also impairs vesicle exocytosis and genetically interacts with vesicle fusion machinery. Therefore, separase may also cleave a substrate to promote exocytosis during CGE and cytokinesis.
Results
SEP-1PD::GFP inhibits chromosome separation
To investigate the proteolytic functions of separase in C. elegans, we used the pie-1 promoter for germline expression of a protease-dead separase (C1040S) fused to GFP (SEP-1PD::GFP).Citation29,38 We have devised two methods to propagate animals carrying the dominant negative protease dead separase and have applied them to characterize the phenotype caused by stable expression of protease-dead separase.Citation29 Depending on the experimental setup and desired genotype, our conditions lead to SEP-1PD::GFP expression from either one or two copies of the transgene in a wild type background with endogenous separase expression. We also characterized multiple independently generated homozygous SEP-1PD::GFP transgenic lines obtained by microparticle bombardment to identify the most reproducibly behaved lines. Two lines (WH520 and WH524) behave as chromosomal-integrated alleles with consistent expression of the protease-dead separase that lead to consistent phenotypes, while other lines were less consistent (Fig. S1A). We used WH520 to characterize cellular phenotypes, which has nearly 100% embryo lethality after 5 generations off GFP RNAi (which we will call homozygous SEP-1PD::GFP) and about 70% lethality in F2 embryos using the backcross propagation strategy (labeled as SEP-1PD::GFP/+) (Fig. S1B). In contrast, expression of SEP-1WT::GFP causes no lethality and can fully rescue mutant separase embryos.Citation29,38 Therefore, expression of SEP-1PD::GFP in the wild type background with endogenous separase consistently causes embryo lethality.
Separase is well known to cleave cohesin to allow chromosome segregation. We hypothesized that SEP-1PD::GFP is dominant negative in part because it may bind cohesin but would be unable to cleave it, thus preventing endogenous separase from cleaving cohesin and inhibiting chromosome separation. Separase has several conserved substrates that are found in C. elegans and mammalian cells, including cohesin. Prior to anaphase onset, SEP-1WT::GFP and SEP-1PD::GFP show identical localization patterns and both show equivalent localization to chromosomes.Citation38 However, in mitotic anaphase, when separase becomes catalytically active and would bind to substrates, SEP-1PD::GFP displays ectopic localization at centrioles and the central spindle where known substrates are cleaved by separase in other systems,Citation38 consistent with the hypothesis that it has enhanced association with substrates. To investigate the effects of SEP-1PD::GFP on chromosome segregation, we compared embryos expressing H2B::mCherry to label the chromosome and homozygous SEP-1PD::GFP or SEP-1WT::GFP. We defined anaphase onset as the time point when the width of the chromosome signal increases due to spindle forces pulling sister chromatids apart, which always occurs very quickly after chromosome alignment on the metaphase plate in both SEP-1PD::GFP and SEP-1WT::GFP. Consistent with our hypothesis, chromosome segregation defects were observed during the first mitosis in homozygous SEP-1PD::GFP compared with SEP-1WT::GFP embryos (). To ensure cell cycle timing was not dramatically altered, we quantified the time from nuclear envelop breakdown (NEBD) to furrow ingression in homozygous SEP-1PD::GFP embryos and did not observe a significant delay of global cell cycle events as compared with SEP-1WT::GFP (Fig. S1C-H, p = 0.54, t-test).
Figure 1. SEP-1PD::GFP causes chromosome segregation defects during mitosis. Representative images of mitotic chromosome segregation in SEP-1WT::GFP expressing embryos (A-D, green) or homozygous SEP-1PD::GFP (green) embryos with slight bridging (E-H) and severe bridging (I-L) co-expressing H2B::mCherry (red). (M) Average distance between separating sister chromatids (as shown by arrowheads in B, F, J) during anaphase in SEP-1WT::GFP (n = 7) or SEP-1PD::GFP (n = 9) embryos from metaphase to late cytokinesis. (N) Percentage of embryos displaying normal chromosome separation (blue), slight bridging chromosomes (red) or severe chromosome bridges (green) during the first mitosis in embryos expressing either SEP-1WT::GFP or SEP-1PD::GFP at the temperature indicated (n = number of embryos imaged). Insert shows H2B::mCherry images scored as normal, slight bridging and severe bridging. Scale Bars, 10 μm. P-values: * = <0.05; **** = <0.0001 (t-test). Error bars indicated standard deviation of the mean.
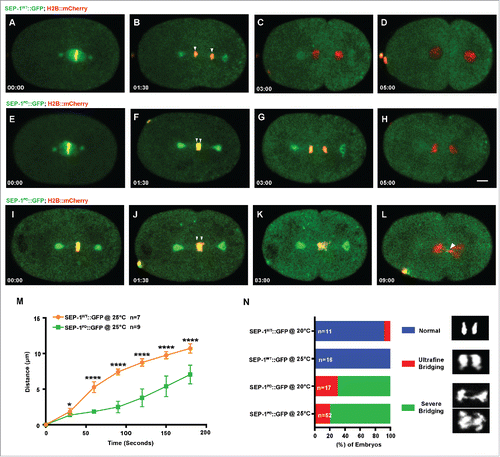
Quantification of the distance that chromosomes separate after mitotic anaphase onset showed on average a 3.7 micron lag in separation over time in embryos expressing homozygous SEP-1PD::GFP (). Homozygous SEP-1PD::GFP embryos had some variation in the severity of segregation defects, from slight bridging (in 10/52 SEP-1PD::GFP embryos at 25°C) to more severe bridging chromosomes (in 42/52 homozygous SEP-1PD::GFP embryos at 25°C) (), which was absent from WT (in 0/16 SEP-1WT::GFP embryos at 25°C). Interestingly, the delayed chromosome separation was more severe at 25°C than at 20°C ( & Movie 1), which is likely due to higher transgene expression (fluorescence intensity in the cytoplasm is twofold higher at 25°C as compared with 20°C). We also investigated chromosome segregation during anaphase I of meiosis. Interestingly, homozygous SEP-1PD::GFP embryos also displayed chromosome segregation defects during meiotic anaphase (). We measured the delay in separation over time and observed a less severe but significant delay in chromosome segregation (). In addition, the bridging defects were not as severe as observed in mitosis (bridge observed in 0/8 SEP-1WT::GFP embryos at 25°C; in 7/15 homozygous SEP-1PD::GFP at 25°C, & Movie 2). These data indicate that homozygous SEP-1PD::GFP impairs chromosome segregation during both meiosis and mitosis, likely due to impaired cohesin cleavage.
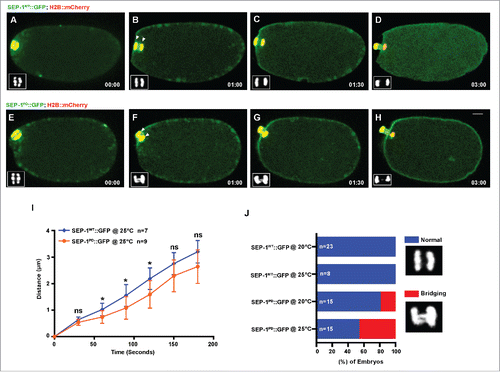
Figure 2. SEP-1PD::GFP causes chromosome segregation defects during meiosis I. Representative images of meiotic chromosome segregation in SEP-1WT::GFP (A-D, green) or homozygous SEP-1PD::GFP expressing embryos (E-H, green) co-expressing H2B::mCherry (red). Lower left insets show H2B::mCherry. (I) Average distance between chromosomes (indicated by arrowheads in B, F) during anaphase in SEP-1WT::GFP or homozygous SEP-1PD::GFP. (J) Percentage of embryos displaying normal chromosome separation (blue), bridging chromosomes (red) during the anaphase I in embryos expressing either SEP-1WT::GFP or homozygous SEP-1PD::GFP (n = number of embryos imaged). Insets show examples scored as normal or bridging chromosomes during anaphase I. Scale Bars, 10 μm. P-values: * = <0.05; ns = not significant (t-test). Error bars indicated standard deviation of the mean.
If our hypothesis that cohesin cleavage is impaired by SEP-1PD::GFP is correct, we would expect that partial depletion of scc-1 by RNAi would alleviate the chromosome segregation defects. We carefully titrated the degree of RNAi depletion (feeding RNAi 24 hours at 20°C and 25°C) to achieve a mild level of scc-1 depletion to avoid causing severe chromosome segregation defects due to loss of cohesin.Citation35 At both 20°C and 25°C, scc-1 RNAi causes only mild lethality in wild type (, ) but significantly rescues the homozygous SEP-1PD::GFP embryonic lethality from 100% down to 22% ± 10.34 at 25°C (). Chromosome segregation defects were significantly alleviated after depletion of scc-1 (RNAi) in homozygous SEP-1PD::GFP embryos (). Homozygous SEP-1PD::GFP depleted of scc-1 also had normal kinetics of chromosome segregation in anaphase () and much less severe bridging defects (28/42 normal, 9/42 slightly bridging, 5/42 severe bridges, ). Therefore, reducing the amount of cohesin largely rescues the chromosome segregation defects caused by expressing SEP-1PD::GFP together with endogenous separase in C. elegans. Presumably this is because there is less substrate that must be cleaved, reducing the amount of cohesin that endogenous separase must cleave in the presence of SEP-1PD::GFP to allow chromosome segregation. These findings suggest that SEP-1PD::GFP acts as a substrate trapping enzyme and inhibits cleavage of cohesin to impair chromosome segregation, as expected from the known functions of separase. Additionally, the data consistent with our hypothesis that SEP-1PD::GFP inhibits substrate cleavage, causing a dominant phenotype.
Figure 3. Cohesin depletion rescues chromosome segregation defects caused by SEP-1PD::GFP. (A, B) Partial cohesin depletion significantly rescues the SEP-1PD::GFP embryonic lethality at both 20°C and 25°C (n = singled worm number: total embryo count). (C-F) Chromosome segregation defects were significantly alleviated after partial depletion of scc-1 in homozygous SEP-1PD::GFP (green) embryos (DNA in red). (G) Distance between separating sister chromatids during anaphase in SEP-1WT::GFP or SEP-1PD::GFP control or with scc-1 (RNAi). (H) Percentage of embryos displaying normal chromosome separation (blue), slight bridging chromosomes (red) or severe chromosome bridges (green) during the first mitosis in embryos expressing SEP-1WT::GFP or SEP-1PD::GFP with and without scc-1 (RNAi) treatment (n = number of embryos imaged). Scale Bars, 10 μm. P-values: ** = <0.01; *** = <0.001; **** = <0.0001 (t-test). Error bars indicated standard deviation of the mean.
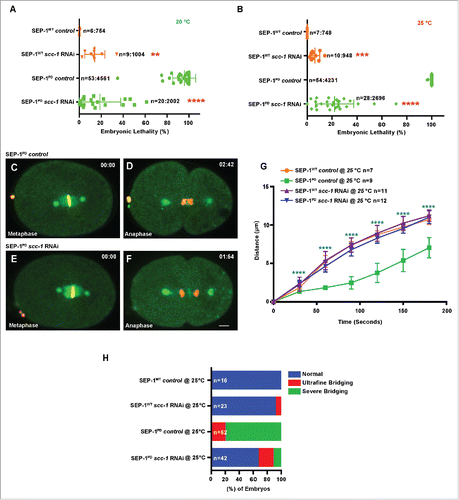
Figure 4. SEP-1PD::GFP causes cytokinesis defects. (A) Percentage of embryos displaying normal cell division (blue) and cytokinesis failure (red) during first mitotic division in N2 wild type, SEP-1WT::GFP or homozygous SEP-1PD::GFP. Right panels show examples scored as normal or cytokinesis failure. (B) Quantification of the furrow ingression rate in different genotypes. Depletion of SCC-1 in SEP-1PD::GFP embryos does not rescue the slower furrow ingression (p = 0.29 (t-test), n = number of embryos imaged). (C) Quantification of the furrow ingression time in different conditions as indicated (n = number of embryos imaged). (D) Kymograph of the furrow region shows PH::mCherry (red) in SEP-1WT::GFP, homozygous SEP-1PD::GFP, SEP-1PD::GFP; scc-1(RNAi) (time in seconds indicated below), or AIR-2::GFP (green) expressing PH::mCherry (red) and H2B::mCherry (red) with and without top-2 (RNAi) during cytokinesis. Distance between separating sister chromatids at similar times after anaphase onset is indicated by brackets, furrow SEP-1PD::GFP signal is indicated by arrowheads. Cohesin depletion rescues chromosome segregation, but not furrowing. The lower kymograph of an embryo treated with top-2 (RNAi) has chromatin in the path of the furrow without any change in furrow ingression. Scale Bars, 10 μm. Error bars indicated standard deviation of the mean. Each kymograph image is 6 seconds apart. P-values: *** = <0.001; **** = <0.0001 (t-test).
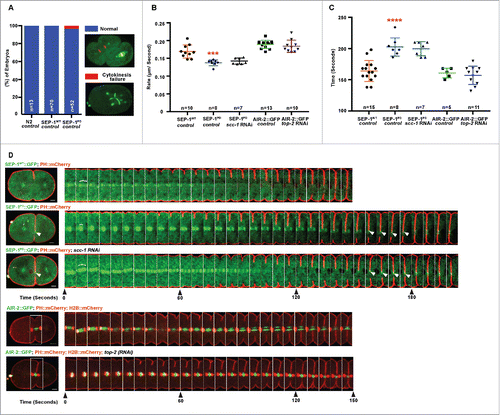
Figure 5. SEP-1PD::GFP was enhanced by t-SNARE syx-4 depletion. (A) Representative images of mitotic cytokinesis in SEP-1WT::GFP (A, green) or homozygous SEP-1PD::GFP (B, green) embryos co-expressing PH::mCherry (red). (B) Representative images of mitotic cytokinesis failure in homozygous SEP-1PD::GFP; PH::mCherry expressing embryos with syx-4 (RNAi), resulting in a one cell embryo with a multi-polar spindle. (C) Percentage of embryos displaying normal cytokinesis (blue) or cytokinesis failure (red) in different conditions as indicated (n = number of embryos imaged). Scale Bars, 10 μm.
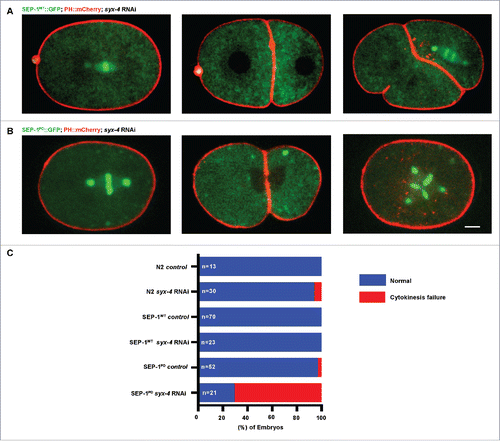
Figure 6. SEP-1PD::GFP inhibits RAB-11 positive vesicle trafficking during cytokinesis. (A, B) Representative images and kymograph of RAB-11::mCherry (red) trafficking to the furrow in SEP-1WT::GFP (green) or heterozygous SEP-1PD::GFP/+ (green). Arrowheads denote enhanced RAB-11::mCherry (gray) accumulation. (C, D) syx-4 (RNAi) enhances RAB-11::mCherry (gray) in both SEP-1WT::GFP and SEP-1PD::GFP/+ at the furrow and midbody. (E) Kymograph of the furrow region showing that RAB-6::mCherry (red) and SEP-1WT::GFP (green) do not accumulate in the furrow. (F) Accumulation of heterozygous SEP-1PD::GFP/+ (green) is observed at the furrow and midbody, but not RAB-6::mCherry (red). (G) Quantification of separase and RAB-11 signals in the midbody during cytokinesis in different conditions as indicated. (H) The percentage of embryos displaying cytokinesis failure in heterozygous SEP-1PD::GFP/+ (green) embryos expressing RAB-11::mCherry/+ (red) with indicated conditions. Scale Bars, 10 μm. P-values: * = <0.05; ** = <0.01; **** = <0.0001 (t-test). Error bars indicated standard error of the mean. Each kymograph image is 6 seconds apart.
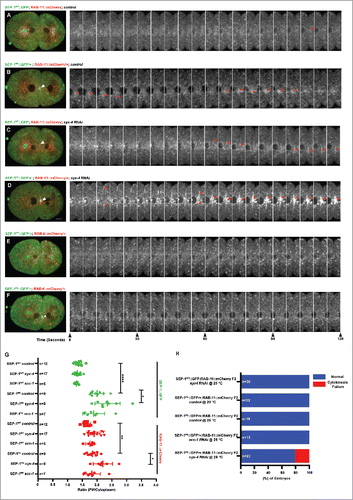
Figure 7. SEP-1PD::GFP expression delays cortical granule exocytosis. (A-F) Representative images of separase localization during anaphase I. Localization of SEP-1WT::GFP (A, green) and SEP-1PD::GFP (D, green) to cortical granules indicated by white arrowheads (H2B::mCherry in red). CGE was delayed in homozygous SEP-1PD::GFP (E) compared with SEP-1WT::GFP (B) during late anaphase I. (F) SEP-1PD::GFP associated with the cortex for a longer time after CGE compared with SEP-1WT::GFP (C). (G) Quantification of anaphase onset to completion of CGE. SEP-1PD::GFP embryos take longer to finish CGE than SEP-1WT::GFP. (H-J) Colocalization of SEP-1PD::GFP (green) with PH::Cherry (red) at the plasma membrane after CGE. (K) Average time that SEP-1WT::GFP or SEP-1PD::GFP remains associated with the plasma membrane after CGE. (L) Ratio of plasma membrane to cytoplasmic SEP-1PD::GFP and SEP-1WT::GFP after onset of anaphase I. Scale Bars, 10 μm. P-values: * = <0.05; *** = <0.001; **** = <0.0001; ns = not significant (t-test). Error bars indicated standard error of the mean.
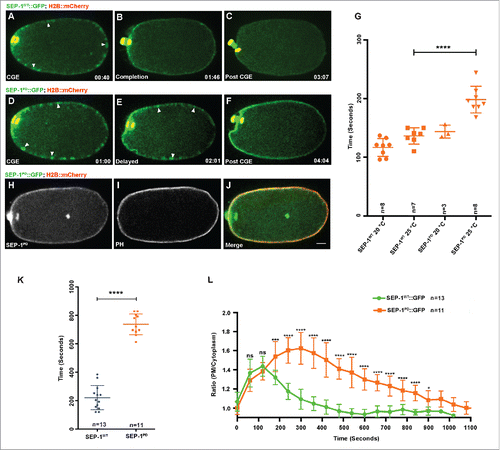
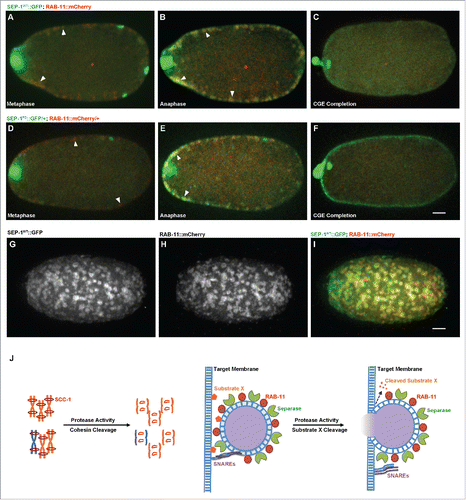
Figure 8. SEP-1PD::GFP does not affect RAB-11 after cortical granule exocytosis. Representative images of meiosis I in embryos expressing separase (green) and RAB-11 (red). (A, D) RAB-11 localizes to cortical granules several minutes before anaphase, before either SEP-1WT::GFP (B, green) or SEP-1PD::GFP/+ (E, green) localize to cortical granules. SEP-1WT::GFP (B, green) and SEP-1PD::GFP (E, green) colocalize with RAB-11::mCherry (red) on the cortical granules in anaphase I. White arrowheads denote colocalization of separase and RAB-11 on cortical granules. (C, F) After exocytosis, SEP-1PD::GFP/+ associated with the plasma membrane while SEP-1WT::GFP and RAB-11::mCherry rapidly disappeared. (G-I) Surface plane of SEP-1WT::GFP (G) and RAB-11::mCherry (H) clearly shows their colocalization (merge in I) on cortical granules. (J) Working model of separase function in exocytosis during cytokinesis. Separase cleaves cohesin kleisin subunit SCC-1 during mitotic anaphase and promotes chromosome segregation. In cytokinesis, separase colocalizes with RAB-11 vesicles. SNAREs including SYX-4 promote vesicle fusion with target membrane. Our results suggest that separase cleaves an unknown substrate to promote exocytosis. Scale Bars, 10 μm.
Current studies indicate that separase cleaves substrates such as kendrin and cohesin to sever the physical link between centrioles.Citation12,41,42 We hypothesized that SEP-1PD::GFP may bind to potential substrates at the centrosome, delaying their cleavage by endogenous separase and inhibiting centriole disengagement. To investigate the effects of SEP-1PD::GFP on centriole disengagement, we compared embryos expressing SPD-2::mCherryCitation43 to label the centrioles and homozygous SEP-1PD::GFP or SEP-1WT::GFP (Fig. S2A, B and Movie 3). We measured the signal intensity of separase in SEP-1PD::GFP and SEP-1WT::GFP expressing embryos at the onset of furrow ingression, which is about the time that centrioles disengage in the AB daughter cell. Interestingly, SEP-1PD::GFP signal is significantly higher at the centriole and centrosome over time, relative to SEP-1WT::GFP embryos (Fig. S2C). However, we did not observe any significant delays in disengagement of daughter centrioles in SEP-1PD::GFP embryos (Fig. S2D). These data suggest that chromosome segregation is more sensitive to inhibition by the protease dead separase than centriole disengagement. Therefore, separase regulates multiple cell cycle events, which have different sensitivity to inhibition by protease dead separase.
SEP-1PD::GFP expression impairs cytokinesis independent of cohesin
In addition to the canonical function of separase in chromosome segregation, separase is required for cytokinesis by regulating vesicle exocytosis.Citation38 If separase has a substrate that it must cleave to promote vesicle exocytosis during cytokinesis, we postulated that SEP-1PD::GFP would inhibit this process similar to the way it impairs chromosome segregation. We tested whether homozygous SEP-1PD::GFP embryos fail cytokinesis using live imaging. Interestingly, we found some homozygous SEP-1PD::GFP embryos with multipolar spindles, indicative of cytokinesis failure, in one cell through 2 cell stages (in 2/52 homozygous SEP-1PD::GFP embryos; in 0/70 SEP-1WT::GFP embryo; in 0/13 N2 at 25°C. ). Additionally, cytokinesis failures are sporadic and are often seen in older SEP-1PD::GFP but not SEP-1WT::GFP embryos, but are difficult to quantify accurately because cells that fail cytokinesis subsequently undergo multipolar division and cellularize. These data indicate that SEP-1PD::GFP expression impairs cytokinesis, consistent with the hypothesis that it may inhibit cleavage of a substrate necessary for cytokinesis.
Next, we analyzed the rate of furrow ingression to determine if there are additional defects during cytokinesis despite the low rate of cytokinesis failure. We generated homozygous SEP-1PD::GFP and SEP-1WT::GFP lines expressing mCherry fused to the pleckstrin homology domain of phospholipase C-delta (PH::mCherry for short) to observe the plasma membrane during cytokinesis.Citation44 We imaged furrow ingression in a single focal plane of the central spindle and midbody. We found that furrow ingression rate in homozygous SEP-1PD::GFP embryos was consistently slower compared with the SEP-1WT::GFP and AIR-2::GFP control (0.14 μm/second, n = 8 in homozygous SEP-1PD::GFP; 0.17 μm/second ± 0.01 n = 10 in SEP-1WT::GFP, p = 0.0004 (t-test), , ). We also measured the time from the initiation of furrow ingression until it completed, generating a smooth cell boundary. In SEP-1WT::GFP cells, this process took 164 seconds ± 4 (n = 15, ). Since SEP-1WT::GFP does not label the midbody, we also imaged the midbody maker AIR-2::GFP together with PH::mCherry and found that our measurement of furrow completion timing was accurate (161 seconds ± 4, n = 5, p = 0.69 (t-test), , ). SEP-1PD::GFP, but not SEP-1WT::GFP, is often colocalized with the plasma membrane during furrowing and remains at the midbody for an extended period of time, which could reflect enhanced association with a membrane substrate (). In homozygous SEP-1PD::GFP embryos, cytokinesis completion was significantly delayed relative to wild type embryos (203 seconds ± 5; n = 8, p<0.0001 (t-test), ). Therefore, expression of dominant negative SEP-1PD::GFP specifically impairs furrow ingression and completion of cytokinesis.
In several systems, lagging chromatin that becomes trapped in the midbody during cytokinesis triggers an “abscission checkpoint” pathway to prevent cytokinesis failure.Citation45,46 In human cells, chromatin bridges induce a delay in abscission but ultimately cells fail cytokinesis, which is observed when cohesin cleavage is impaired.Citation2,47 However whether this is also due to membrane trafficking defects is unknown. Several observations suggest that the cytokinesis defects in SEP-1PD::GFP embryos are different than those caused by other chromosome bridging conditions. First, more penetrant cohesin scc-1 RNAi causes severe chromosome segregation defects but no cytokinesis defects, suggesting that bridges resulting from the cohesin depletion do not cause cytokinesis failure in the embryo.Citation35 In addition, we previously demonstrated that many different types of chromosome defects such as decondensation or catenation cause severe bridging phenotypes but very rare cytokinesis failures due to the action of an abscission checkpoint pathway in C. elegans.Citation38,45 Therefore, chromatin bridges do not cause cytokinesis defects in C. elegans, but elicit the abscission checkpoint, which reduces the failure rate. Consistent with this, we measured the furrow ingression rate in embryos with chromatin bridges after depletion of top-2 and observed normal ingression furrow rate (0.18 ± 0.01, n = 10, p = 0.40 (t-test), ), suggesting the abscission checkpoint does not affect the rate of furrowing like SEP-1PD::GFP. Reports in other systems have indicated that the abscission checkpoint regulates other cytoskeletal regulators that function during cytokinesis.Citation48 Therefore, the abscission checkpoint is likely independent of separase-regulated cytokinesis events.
Cohesin is the critical target of separase in chromosome segregation and is also found on the centrosome where it is cleaved during centriole licensing.Citation42 A function for cohesin during cytokinesis has not been previously reported. If cohesin were the relevant substrate involved in cytokinesis, even at a lower threshold, we would expect its depletion to reduce the amount of substrate necessary to be cleaved and alleviate the cytokinesis defects. However, while 70% of the SEP-1PD::GFP embryos treated with scc-1(RNAi) are rescued for the chromosome segregation defects (), they still show slow furrow ingression and delayed closure of the furrow (). Partial depletion of scc-1 rescues the chromosome segregation defects but did not rescue the delay of furrow closure (200 seconds ± 4, n = 7, p = 0.69 (t-test), , , S3C) or the furrow ingression rate in homozygous SEP-1PD::GFP embryos (0.14 μm/second in homozygous SEP-1PD::GFP scc-1 RNAi; n = 7, p = 0.29 (t-test), ). Interestingly, we found that cohesin depletion leads to higher accumulation of SEP-1PD::GFP at the furrow and midbody in homozygous embryos (Fig. S3A, B). This result suggests that SEP-1PD::GFP can compete with different substrates and when cohesin is depleted, it is more free to interact with a putative unknown substrate at the furrow and midbody. Therefore, the cytokinesis defects observed in embryos expressing SEP-1PD::GFP does not occur in other chromosome bridging conditions and is not rescued by depletion of cohesin, suggesting that separase has a chromosome independent role in cytokinesis.
We further investigated whether cohesin alleviates the cytokinesis defects caused by inactivating separase. We depleted separase by RNAi with and without cohesin depletion to determine whether cohesin depletion would impact the cytokinesis phenotype. To obtain consistent phenotypes, we carefully titrated the degree of RNAi depletion of cohesin and separase (feeding scc-1 RNAi 24 hours and sep-1 RNAi together with scc-1 RNAi for another 24 hours at 20°C). However, depletion of scc-1 did not affect the rate of cytokinesis failure after separase depletion (11/43 sep-1 (RNAi), 12/42 sep-1; scc-1(RNAi), Fig. S3D). We also depleted scc-1 in the hypomorphic separase temperature sensitive mutant (feeding scc-1 RNAi for 48 hours at 15°C), sep-1 (e2406) shifted to 25°C for 4–8 hours and saw no change in the rate of cytokinesis failure (3/10 control (RNAi); sep-1 (e2406), 4/15 scc-1 (RNAi); sep-1(e2406), Fig. S3D). Therefore, cohesin depletion does not affect the cytokinesis defects caused by disrupting separase function in 3 different conditions, suggesting that separase has another substrate besides cohesin that it cleaves to promote cytokinesis.
SEP-1PD::GFP genetically interacts with essential exocytosis machinery
Given that separase likely regulates cytokinesis by promoting RAB-11 vesicle exocytosis, we investigated whether SEP-1PD::GFP interferes with exocytosis. We first tested whether there was a genetic interaction between SEP-1PD::GFP and the t-SNARE syx-4. SYX-4 is a core part of the exocytosis fusion machinery and is localized to the plasma membrane where it is required for cytokinesis in C. elegans.Citation49 Therefore, we expected that combining SEP-1PD::GFP expression and depletion of syx-4 would greatly exacerbate the cytokinesis failure rate if they both inhibit exocytosis. syx-4 RNAi is inefficient and causes highly variable phenotypes compared with other genes.Citation49 We carefully calibrated RNAi treatment and determined that 30–36 hours feeding syx-4 RNAi was an optimal intermediate condition, which caused minimal eggshell permeability and cytokinesis defects in wild type embryos. Consistent with our hypothesis, 30–36 hours feeding syx-4 RNAi synergistically enhanced embryonic cytokinesis defects in embryos expressing homozygous SEP-1PD::GFP (in 15/21 cytokinesis failure, , ) as compared with wild type (in 0/23 SEP-1WT::GFP embryos; in 2/30 N2 embryos, , , Movie 4). Therefore, SEP-1PD::GFP has a strong negative genetic interaction with syx-4(RNAi), consistent with the hypothesis that they both inhibit exocytosis during cytokinesis.
SEP-1PD::GFP inhibits RAB-11 positive vesicle trafficking during cytokinesis
We next wanted to investigate whether the cytokinesis defects caused by SEP-1PD::GFP expression were due to the inhibition of RAB-11 positive vesicle trafficking. Despite several attempts we were unable to generate viable lines homozygous for both SEP-1PD::GFP and RAB-11::mCherry, indicative of a negative genetic interaction. However, we could generate viable heterozygous SEP-1PD::GFP/+ and RAB-11::mCherry/+ F1 animals that reproducibly expressed both transgenes to film F2 embryos. Since the protein in newly fertilized F2 embryos is synthesized by the F1 maternal syncytial germline, each embryo will have the same cytoplasmic expression of SEP-1PD::GFP/+ and RAB-11::mCherry/+ despite having different genotypes. Although the cytokinesis phenotypes in SEP-1PD::GFP/+ expressing RAB-11::mCherry/+ are less severe than homozygous SEP-1PD::GFP, 30–36 hours feeding of syx-4 RNAi substantially increased the rate of cytokinesis failures (0/30 syx-4(RNAi); SEP-1WT::GFP, 0/15 SEP-1PD::GFP/+, 5/23 syx-4(RNAi); SEP-1PD::GFP/+, and Movie 5). Mounting embryos on an agar pad or in hanging drop gave the same results after treating syx-4 RNAi in SEP-1PD::GFP compared with SEP-1WT::GFP embryos, indicating that indirect effects from mounting were not an issue. Therefore, syx-4 RNAi strongly exacerbates the cytokinesis defects in both heterozygous and homozygous SEP-1PD::GFP embryos, although the cytokinesis phenotypes are weaker in the heterozygous embryos.
Next, we imaged RAB-11 vesicle trafficking during cytokinesis in SEP-1WT::GFP and SEP-1PD::GFP/+ embryos. RAB-11 generates exocytic vesicles from recycling endosomes at the centrosome, and remains associated with those vesicles as they are transported to and exocytosed at the plasma membrane.Citation50-52 In SEP-1WT::GFP and SEP-1PD::GFP/+ embryos, RAB-11 is normally distributed at centrosomes and throughout the cytoplasm, indicating that early stages of vesicle trafficking are normal (, ). Interestingly, we found that the expression of SEP-1PD::GFP/+ resulted in increased and persistent accumulation of RAB-11 vesicles at the cleavage furrow and midbody compared with SEP-1WT::GFP expressing embryos, consistent with a defect in exocytosis at the plasma membrane (, , ; Movie 6). The Golgi-associated GTPase, RAB-6, was shown to recruit separase to the cortical granule in C. elegans embryos.Citation53 However, we did not observe the accumulation of RAB-6 at the ingressing furrow or midbody during cytokinesis in SEP-1PD::GFP/+ expressing embryos (, ). Therefore, SEP-1PD::GFP/+ interferes with RAB-11 trafficking during cytokinesis.
Given that SEP-1PD::GFP expression combined with syx-4 (RNAi) enhances cytokinesis failure (), we hypothesized that they both inhibit RAB-11 vesicle exocytosis. To examine this further, we examined whether RAB-11 trafficking was more defective in SEP-1PD::GFP/+; syx-4 (RNAi) embryos, which might explain the increased cytokinesis failure. We imaged RAB-11 vesicles in embryos expressing both RAB-11::mCherry/+ and SEP-1PD::GFP/+ with and without 30–36 hours feeding syx-4 RNAi treatment. Depletion of syx-4 caused a significantly higher accumulation of both RAB-11::mCherry and SEP-1PD::GFP/+ at the ingressing furrow and midbody compared with untreated SEP-1PD::GFP/+ embryos (, , ; Movie 6). Unfortunately we could not assay RAB-11 vesicle trafficking under the more severe condition of homozygous SEP-1PD::GFP; syx-4 (RNAi) which has a much higher cytokinesis failure rate, but we expect that RAB-11 accumulation would be even greater. These data are consistent with the hypothesis that separase and RAB-11 are trafficked together on vesicles to the plasma membrane during cytokinesis, and that syx-4(RNAi) delays fusion of these vesicles. Finally, we examined whether cohesin would cause any change in RAB-11 vesicle trafficking. Given that partial depletion of scc-1 does not significantly change the rate of furrow ingression in SEP-1PD::GFP embryos (), we expected RAB-11 trafficking would also not be affected. Indeed, depletion of SCC-1 did not alter the accumulation of RAB-11 vesicles at the furrow in SEP-1PD::GFP/+ embryos, but rescued the chromosome segregation defect (p = 0.90, t-test, ). Importantly, we previously demonstrated that depletion of top-2, which causes severe chromosome bridging and activates the abscission checkpoint response in C. elegans, does not have any impact on RAB-11 trafficking.Citation38,45 Therefore, the response to chromosome bridging during cytokinesis does not explain the defects in RAB-11 trafficking in SEP-1PD::GFP embryos. These results suggest that separase regulates cytokinesis by hydrolyzing an unknown substrate to regulate RAB-11 vesicle trafficking.
SEP-1PD::GFP expression delays cortical granule exocytosis
Separase and RAB-11 both localize to cortical granules while SYX-4 localizes to the plasma membrane to promote their exocytosis during meiosis anaphase I.Citation36,37,49 This is an excellent cellular context to investigate exocytosis because separase can be observed directly on these large 1μm vesicles, which release contents required for eggshell formation during anaphase I. We investigated whether SEP-1PD::GFP also impairs CGE similar to its effects during cytokinesis. We analyzed whether embryos were permeable to dyes due to disrupted eggshell formation from lack of CGE, but did not observe significant permeability defects. This indicates that SEP-1PD::GFP expression does not completely inhibit CGE. To confirm localization, we filmed SEP-1PD::GFP/+ embryos expressing the cortical granule cargo, CPG-2::mCherry, during anaphase I.Citation54 We observed that CGP-2::mCherry localizes to cortical granules with both SEP-1WT::GFP and SEP-1PD::GFP/+ as expected (Fig. S4 and Movie 7). Interestingly, separase localizes to more vesicles than those labeled by CPG-2::mCherry, indicating that this cargo is only packaged into a subset of cortical granules (Fig. S4, Movie 7). This result is consistent with the heterogeneity of the cortical granule vesicle population observed by transmission electron microscope.Citation36
Next, we investigated whether CGE was delayed in homozygous SEP-1PD::GFP embryos relative to SEP-1WT::GFP. We imaged anaphase I with H2B::mCherry and SEP-1::GFP to observe both chromosomes and cortical granules and quantified the time from anaphase onset until CGE completion during anaphase I. CGE was significantly delayed in homozygous SEP-1PD::GFP expressing embryos (198 seconds ± 8, n = 8) compared with SEP-1WT::GFP expressing embryos (136 seconds ± 5, n = 7, p<0.0001, t-test) at 25°C ( and Movie 8). In addition, we observed that SEP-1PD::GFP remained associated with the plasma membrane for a longer time after CGE (736 seconds ± 22, n = 11) compared with SEP-1WT::GFP (221 seconds ± 24, n = 13, p<0.0001, t-test) ( & Movie 9). Quantification of the plasma membrane localized signal shows that both SEP-1WT::GFP and SEP-1PD::GFP initially accumulate on the membrane to similar amounts, but SEP-1PD::GFP accumulates to a higher level and remains associated with the membrane for substantially longer (). These data are consistent with the hypothesis that SEP-1PD::GFP may block cleavage of putative substrate involved in exocytosis and that it may remain bound to a substrate after exocytosis in the plasma membrane.
SEP-1PD::GFP does not affect RAB-11 after cortical granule exocytosis
RAB-11 localizes to cortical granules and is required for CGE.Citation37 Therefore, we investigated whether SEP-1PD::GFP affects the dynamics of RAB-11 during and after CGE. We filmed meiotic stage embryos expressing SEP-1::GFP/+ and RAB-11::mCherry and observed that RAB-11::mCherry localizes to cortical granules several minutes before anaphase,Citation37 before either SEP-1WT::GFP or SEP-1PD::GFP/+ localize to cortical granules (, ). Just after anaphase onset, before exocytosis, both forms of separase fully co-localize with all RAB-11::mCherry labeled cortical granules before exocytosis (, , ). Therefore, RAB-11 and separase are localized to the same population of CGs and are sequentially recruited to cortical granules through an orderly process leading to exocytosis in anaphase I (Movie 9). After exocytosis, SEP-1PD::GFP/+ associated with the plasma membrane for an extended time while RAB-11::mCherry rapidly disappeared ( and Movie 10). This result suggests that SEP-1PD::GFP/+ does not require RAB-11 to remain associated with the plasma membrane, but might bind another unknown substrate. Therefore, RAB-11 and separase may function in parallel but independent pathways to promote exocytosis during anaphase.
Discussion
The mechanism by which separase regulates chromosome segregation is well known, while its function in exocytosis during CGE and cytokinesis needs to be elucidated. Here, we explore whether the proteolytic activity of separase is involved in its membrane trafficking roles. Utilizing our novel observation that protease dead separase is dominant negative, we provide data showing that it interferes with endogenous separase function during chromosome segregation and cytokinesis. Therefore, we hypothesize that separase uses its protease activity to cleave cohesin to allow chromosome segregation and to independently cleave multiple other substrates to promote several events during anaphase, including membrane trafficking during cytokinesis.
During chromosome segregation, the well-established function of separase is to cleave the cohesin subunit SCC-1 during mitosis. Consistent with the hypothesis that SEP-1PD::GFP impairs substrate cleavage by the endogenous separase, we observe chromosome segregation defects in SEP-1PD::GFP expressing embryos. Furthermore, depletion of SCC-1 substantially recues mitotic chromosome segregation and embryo lethality caused by SEP-1PD::GFP. Previously, SCC-1 was not detected on chromosomes after prophase, suggesting that separase may not cleave cohesin to promote the metaphase to anaphase transition.Citation35 However, our results are consistent with the hypothesis that separase is required to cleave whatever remaining cohesin is present on metaphase chromosomes for proper segregation to occur at anaphase onset.
Whether separase has a substrate involved in exocytosis is unknown. However, we find that the protease-dead separase causes cytokinesis failure and inhibits RAB-11 vesicle exocytosis during mitotic cytokinesis. These data are consistent with a model whereby separase cleaves a substrate to promote exocytosis (), similar to its function during chromosome segregation. On its own, SEP-1PD::GFP does not cause a severe cytokinesis defect, but synergistically inhibits cytokinesis when syx-4 is depleted, while chromosome segregation is more obviously defective. It is worth nothing that C. elegans centromeres are holocentric,Citation55 meaning that cohesin must be cleaved along the entire chromosome instead of a point centromere as in other organisms and thus chromosome segregation could be more sensitive to delayed cohesin cleavage. We also did not observe significant defects in centriole disengagement. Given that RAB-11 and endosomes have been observed at centrioles in human cells,Citation56 the enhanced centriole localization of SEP-1PD::GFP may be related to membrane trafficking functions as well as substrates involved in disengagement. Therefore, separase likely cleaves substrates involved in several different process, but the effects imposed by SEP-1PD::GFP vary in different events.
There are several possible explanations for these observations. The first is that our overexpression levels are not high enough to effectively block cleavage of a putative vesicle target, but is sufficient to inhibit chromosome segregation. This could be due to the affinity of separase toward different substrates. Alternatively, protease dead separase may bind to substrates and alter their function independently of cleavage, such as sequestering them from other interactions. Although the precise molecular effect of SEP-1PD::GFP on substrates may be unclear, our results suggest that substrates are involved in various cellular functions of separase including exocytosis. While substrate cleavage may be involved in exocytosis, delayed cleavage may not be sufficient on its own to block exocytosis in the presence of all other factors that promote exocytosis. Consistent with this, depletion of separase does not completely block centriole separation and other factors minimize the resulting phenotypes.Citation34 Certainly the local environment at chromosomes, centrioles and vesicles is quite different. This could impact how stably separase can interact with substrates and thus how well SEP-1PD::GFP can inhibit substrate cleavage. Indeed, separase catalytic activity toward cohesin is much greater in the presence of DNA,Citation57 while the fluid environment of a membrane may not have the same effect. The finding that separase is dramatically stimulated by DNA suggests that both cohesin and separase associate with DNA, increasing the local concentration of both to promote catalysis. We did not observe any loss of separase localization to chromosomes after cohesin depletion, suggesting separase localizes to chromosomes independently of the substrate. In addition to substrate affinity, SEP-1PD::GFP may displace endogenous separase from chromosome more readily than it does in the membrane. Therefore, there may also be differences in the relative amounts of transgenic SEP-1PD::GFP to the amount of endogenous separase at different cellular locations. The relative amounts of endogenous vs. transgenic separase protein may also explain why we generally observed less severe meiotic phenotypes vs. mitotic phenotypes. Future studies will be required to resolve these issues.
While separase is a protease, critical non-proteolytic functions of separase are required for mitotic exit. Previously, three C. elegans separase mutant alleles have been identified, all of which map outside of the protease domain.Citation58 Interestingly, each of these mutants cause defects in cortical granule exocytosis and mitotic cytokinesis failure, but minimal chromosome segregation defects.Citation58 Furthermore, these mutants are rescued by loss of phosphatase 5 (pph-5), which might represent a signaling pathway that controls exocytosis.Citation58 While our results suggest that separase has a substrate involved in exocytosis, we cannot rule out non-proteolytic functions that may also impact exocytosis. For example, Cdk5 is involved in the regulation of synaptic vesicle exocytosis via phosphorylation of munc18.Citation59 Separase may regulate CDK or perhaps another signaling pathway to control exocytosis. Ultimately, separase may have both proteolytic and non-proteolytic functions that collaborate to promote exocytosis during anaphase. This might be required to ensure that separase promotes exocytosis after a significant delay in anaphase, which occurs during both meiosis and mitosis. Elucidating how the precise control of separase function leads to exocytosis during anaphase will be an important goal of future studies.
Our observations show that RAB-11 is recruited to cortical granules much earlier than separase, which suggests an ordered recruitment of regulators to these vesicles before their exocytosis in anaphase. Defining the pathway and signals that control the timing sequence of this recruitment process will be important to better understand how the cell cycle and potentially other pathways coordinate vesicle trafficking during cell division. Whether the same process occurs during mitotic cytokinesis will require much better imaging conditions since the individual vesicles are small and dynamic as they move along the spindle. Interestingly, SEP-1PD::GFP associates with plasma membrane for an extended period of time after cortical granule exocytosis, however, RAB-11 does not. This indicates that RAB-11 is not required for SEP-1PD::GFP to remain associated with the plasma membrane and may not be the substrate of the separase during exocytosis. This result is consistent with previous observations that depletion of RAB-6, but not RAB-11, prevents recruitment of separase to cortical granules.Citation53 It is still possible that separase may cleave RAB-11 interacting proteins. This could indicate that separase affects a different step in exocytosis than the membrane docking and tethering functions mediated by RAB-11. For example, separase might cleave a substrate that allows vesicles to move forward in the exocytosis pathway, i.e., moving from a docked to a primed state.Citation7 The timing when cortical granules undergo different steps of exocytosis in C. elegans is unknown, but it is possible that the early steps are completed by the time that separase is completely transferred to vesicles in anaphase. Indeed, cortical granules in sea urchin have been shown to be in a “hemifusion” state and fertilization happens post anaphase in this organism.Citation15 Separase may cleave RAB-11 interacting proteins, such as RAB-11 GEFs, to regulate RAB-11 activity during exocytosis.Citation60 Another possibility is that separase cleaves an inhibitor of exocytosis, such as the complexin protein that prevents SNAREs from completing vesicle fusion prematurely.Citation41 Identifying a putative vesicle target that separase cleaves to promote exocytosis is a primary pursuit for future investigation. This may provide novel mechanistic insights into how a protease can promote exocytosis, which may also be applicable to membrane trafficking events independent of the cell cycle.
Materials and methods
C. elegans strains
C. elegans strains were maintained with standard protocols, except for the modified procedures to maintain toxic transgenes (below). Strain information is listed in . Some strains used in this study were obtained from the Caenorhabditis Genetics Center (CGC). Strain RQ372 was gift from Dr. Risa Kitagawa. JAB18 was created by crossing WH520 males with OD56 hermaphrodites.Citation29 JAB156 was generated by crossing WH520 males with EKM41 hermaphrodites, and subsequent generations were maintained on gfp RNAi. At F2 generation following the cross, L4 stage worms were singled from the original gfp RNAi feeding plates. We screened the F3 adults for the presence of PH::mCherry transgenes by microscopy. Then approximately half of the PH::mCherry positive worms at L4 stage were moved to OP50 plates for 3–4 generations, and screened for the presence of both transgenes. The protocol was repeated until double homozygous transgenic lines were obtained, after which the line was maintained on gfp RNAi.
Table 1. Strains used in this study.
Propagation of the protease dead separase strains
We demonstrated that SEP-1PD::GFP expression is dominant negative.Citation29 Using this mutant, we have devised two methods to propagate protease dead separase transgenic animals. One method is crossing SEP-1PD::GFP male worms, generated from hermaphrodites on gfp(RNAi) to unc-119 mutant hermaphrodites each generation. Crossing the SEP-1PD::GFP transgene with an unc-119 mutant rescues the movement defect of the F1 animals as the SEP-1PD::GFP construct contains wild type unc-119. The SEP-1PD::GFP transgene is driven by the pie-1 promoter, which is only expressed in the female germline. Therefore, the SEP-1PD::GFP transgene can be propagated in male worms without deleterious effects and the hermaphrodite siblings can be assayed for phenotypes. We backcrossed SEP-1PD::GFP male worms with unc-119 mutant hermaphrodites for at least 8 generations to ensure that generational inheritance of GFP RNAi had been lost. This method reduces background mutations that might complicate phenotypic analysis and allows us to introduce the transgene into backgrounds that we cannot make homozygous. The second method is feeding gfp RNAi to eliminate SEP-1PD::GFP transgene expression. After animals are transferred from gfp RNAi food onto regular bacteria food for 5–6 generations, the inherited RNAi will be lost and transgene expression will occur again.
RNAi treatment
The gfp and syx-4 RNAi feeding constructs were described previously,Citation29,38 and scc-1 RNAi was obtained from the Ahringer library.Citation61 To silence the target genes, L4 hermaphrodites were picked onto lawns of IPTG-induced RNAi feeding bacteria. To provide the optimal RNAi effect for target genes silencing, RNAi cultures were grown till log phase. Then the log phase RNAi bacteria were spread on plates containing NGM agar with 1 mM IPTG and the plates were incubated at 15°C for 24–48 hours to optimally induce the T7 promoter expression.Citation62 Worms were grown on RNAi plates at 20°C /25°C for the amount of time indicated in the manuscript for different experiments.
Microscopy
For live imaging, young adult worms were dissected in M9 buffer and embryos were mounted on agar pads as described previously.Citation29 For imaging of meiotic embryos, or potentially osmotic sensitive embryos, young adults were dissected and mounted in blastomere culture media by hanging drop to relieve mechanical and osmatic pressure.Citation63 Live cell imaging was performed on a spinning disk confocal system that uses a Nikon Eclipse inverted microscope with a 60 × 1.40NA objective, a CSU-22 spinning disk system and a Photometrics EM-CCD camera from Visitech International. Images were acquired by Metamorph (Molecular Devices) and analyzed by ImageJ/FIJI Bio-Formats plugins (National Institutes of Health).Citation64,65
Statistics
Quantification of SEP-1::GFP and RAB-11::mCherry at the midbody was performed in Image J by measuring the fluorescent intensity at the midbody in frames with the brightest signal shortly after furrow ingression was completed. Embryos were shifted to 25°C to improve signal, but caused abnormal aggregates of RAB-11::mCherry in some embryos, which were not included in the analysis. To account for variations in imaging and z-depth, we calculated the ratio of the intensity at the midbody relative to cytoplasm. Cytoplasm signal was determined by averaging the intensities from 3 separate regions in the same image. Statistical significance was determined by p value from an unpaired 2-tailed t-test. P-values: ns = not significant; * = <0.05; ** = <0.01, *** = <0.001; **** = <0.0001. Each data set was evaluated with both the Shapiro-Wilk and Kolmogorov-Smirnov normality tests and all data follow normal distributions.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KCCY_S_1363936_Supplementary_material.zip
Download Zip (53.3 MB)Acknowledgments
We appreciate the CGC (University of Minnesota) funded by the NIH Office of Research Infrastructure Programs (P40 OD010440) which provided some C. elegans strains. We thank Kevin O'Connell for generously sharing SPD-2::mCherry before publication.Citation43 We are grateful to members of the Bembenek laboratory, Michael Melesse, Christopher Turpin, Nicolas Mattson, for productive discussion and preparing reagents. We also thank Bruce McKee, Maitreyi Das, Nasser Rusan and Don Fox for critical feedback on the manuscript. This work was funded by startup funds from UT Knoxville and by NIH R01 GM114471.
References
- Onn I, Heidinger-Pauli JM, Guacci V, Unal E, Koshland DE. Sister Chromatid Cohesion: A Simple Concept with a Complex Reality. Annu Rev Dev Biol. 2008;24:105-29. doi:10.1146/annurev.cellbio.24.110707.175350. PMID:18616427
- Hauf S, Waizenegger IC, Peters JM. Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science. 2001;293:1320-3. doi:10.1126/science.1061376. PMID:11509732
- Boland A, Martin TG, Zhang ZG, Yang J, Bai XC, Chang LF, Scheres SHW, Barford D. Cryo-EM structure of a metazoan separase-securin complex at near-atomic resolution. Nat Struc Mol Biol. 2017;24:414-+. doi:10.1038/nsmb.3386
- Lin Z, Luo X, Yu H. Structural basis of cohesin cleavage by separase. Nature. 2016;532:131-4. doi:10.1038/nature17402. PMID:27027290
- Luo S, Tong L. Molecular mechanism for the regulation of yeast separase by securin. Nature. 2017;542:255-59. doi:10.1038/nature21061. PMID:28146474
- Viadiu H, Stemmann O, Kirschner MW, Walz T. Domain structure of separase and its binding to securin as determined by EM. Nat Struct Mol Biol. 2005;12:552-3. doi:10.1038/nsmb935
- Winter A, Schmid R, Bayliss R. Structural Insights into Separase Architecture and Substrate Recognition through Computational Modelling of Caspase-Like and Death Domains. Plos Comput Biol. 2015;11:1-20. doi:10.1371/journal.pcbi.1004548. PMID:26513470.
- Uhlmann F, Wernic D, Poupart MA, Koonin EV, Nasmyth K. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell. 2000;103:375-86. doi:10.1016/S0092-8674(00)00130-6. PMID:11081625
- Sullivan M, Hornig NC, Porstmann T, Uhlmann F. Studies on substrate recognition by the budding yeast separase. J Biol Chem. 2004;279:1191-6. doi:10.1074/jbc.M309761200. PMID:14585836
- Sullivan M, Lehane C, Uhlmann F. Orchestrating anaphase and mitotic exit: separase cleavage and localization of Slk19. Nat Cell Biol. 2001;3:771-7. doi:10.1038/ncb0901-771. PMID:11533655
- Lee K, Rhee K. Separase-dependent cleavage of pericentrin B is necessary and sufficient for centriole disengagement during mitosis. Cell Cycle. 2012;11:2476-85. doi:10.4161/cc.20878. PMID:22722493
- Matsuo K, Ohsumi K, Iwabuchi M, Kawamata T, Ono Y, Takahashi M. Kendrin Is a Novel Substrate for Separase Involved in the Licensing of Centriole Duplication. Curr Biol. 2012;22:915-21. doi:10.1016/j.cub.2012.03.048. PMID:22542101
- Waizenegger IC, Gimenez-Abian JF, Wernic D, Peters JM. Regulation of human separase by securin binding and autocleavage. Curr Biol. 2002;12:1368-78. doi:10.1016/S0960-9822(02)01073-4. PMID:12194817
- Stemmann O, Zou H, Gerber SA, Gygi SP, Kirschner MW. Dual inhibition of sister chromatid separation at metaphase. Cell. 2001;107:715-26. doi:10.1016/S0092-8674(01)00603-1. PMID:11747808
- Zou H, Stemmann O, Anderson JS, Mann M, Kirschner MW. Anaphase specific auto-cleavage of separase (vol 528, pg 246, 2002). Febs Lett. 2002;531:381-381. doi:10.1016/S0014-5793(02)03547-0
- Holland AJ, Bottger F, Stemmann O, Taylor SS. Protein phosphatase 2A and separase form a complex regulated by separase autocleavage. J Biol Chem. 2007;282:24623-32. doi:10.1074/jbc.M702545200. PMID:17604273
- Papi M, Berdougo E, Randall CL, Ganguly S, Jallepalli PV. Multiple roles for separase auto-cleavage during the G2/M transition. Nat Cell Biol. 2005;7:1029-U144. doi:10.1038/ncb1303. PMID:16138084
- Stegmeier F, Visintin R, Amon A. Separase, polo kinase, the kinetochore protein Slk19 and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell. 2002;108:207-20. doi:10.1016/S0092-8674(02)00618-9. PMID:11832211
- Sullivan M, Uhlmann F. A non-proteolytic function of separase links the onset of anaphase to mitotic exit. Nat Cell Biol. 2003;5:249-54. doi:10.1038/ncb940. PMID:12598903
- Chin CF, Bennett AM, Ma WK, Hall MC, Yeong FM. Dependence of Chs2 ER export on dephosphorylation by cytoplasmic Cdc14 ensures that septum formation follows mitosis. Mol Biol Cell. 2012;23:45-58. doi:10.1091/mbc.E11-05-0434. PMID:22072794
- Jakobsen MK, Cheng ZL, Lam SK, Roth-Johnson E, Barfield RM, Schekman R. Phosphorylation of Chs2p regulates interaction with COPII. J Cell Sci. 2013;126:2151-56. doi:10.1242/jcs.115915. PMID:23525003
- Palani S, Meitinger F, Boehm ME, Lehmann WD, Pereira G. Cdc14-dependent dephosphorylation of Inn1 contributes to Inn1-Cyk3 complex formation. J Cell Sci. 2012;125:3091-96. doi:10.1242/jcs.106021. PMID:22454527
- Miller DP, Hall H, Chaparian R, Mara M, Mueller A, Hall MC, Shannon KB. Dephosphorylation of Iqg1 by Cdc14 regulates cytokinesis in budding yeast. Mol Biol Cell. 2015;26:2913-26. doi:10.1091/mbc.E14-12-1637. PMID:26085509
- Kuilman T, Maiolica A, Godfrey M, Scheidel N, Aebersold R, Uhlmann F. Identification of Cdk targets that control cytokinesis. Embo J. 2015;34:81-96. doi:10.15252/embj.201488958. PMID:25371407
- Gorr IH, Boos D, Stemmann O. Mutual inhibition of separase and Cdk1 by two-step complex formation. Mole Cell. 2005;19:135-41. doi:10.1016/j.molcel.2005.05.022. PMID:15989971
- Gorr IH, Reis A, Boos D, Wuhr M, Madgwick S, Jones KT, Stemmann O. Essential CDK1-inhibitory role for separase during meiosis I in vertebrate oocytes. Nature Cell Biol. 2006;8:1035-U120. doi:10.1038/ncb1467. PMID:16906143
- Hellmuth S, Pohlmann C, Brown A, Bottger F, Sprinzl M, Stemmann O. Positive and Negative Regulation of Vertebrate Separase by Cdk1-Cyclin B1 May Explain Why Securin Is Dispensable. J Biol Chem. 2015;290:8002-10. doi:10.1074/jbc.M114.615310. PMID:25659430
- Kudo NR, Wassmann K, Anger M, Schuh M, Wirth KG, Xu HL, Helmhart W, Kudo H, Mckay M, Maro B, et al. Resolution of chiasmata in oocytes requires separase-mediated proteolysis. Cell. 2006;126:135-46. doi:10.1016/j.cell.2006.05.033. PMID:16839882
- Mitchell DM, Uehlein-Klebanow LR, Bembenek JN. Protease-Dead Separase Is Dominant Negative in the C. elegans Embryo. Plos One. 2014;9:1-9. doi:10.1371/journal.pone.0108188
- McCarter J, Bartlett B, Dang T, Schedl T. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol. 1999;205:111-28. doi:10.1006/dbio.1998.9109. PMID:9882501
- Siomos MF, Badrinath A, Pasierbek P, Livingstone D, White J, Glotzer M, Nasmyth K. Separase is required for chromosome segregation during meiosis I in Caenorhabditis elegans. Curr Biol. 2001;11:1825-35. doi:10.1016/S0960-9822(01)00588-7. PMID:11728305
- Monen J, Hattersley N, Muroyama A, Stevens D, Oegema K, Desai A. Separase Cleaves the N-Tail of the CENP-A Related Protein CPAR-1 at the Meiosis I Metaphase-Anaphase Transition in C-elegans. Plos One. 2015;10:1-16. doi:10.1371/journal.pone.0125382. PMID:25919583
- Schvarzstein M, Pattabiraman D, Bembenek JN, Villeneuve AM. Meiotic HORMA domain proteins prevent untimely centriole disengagement during Caenorhabditis elegans spermatocyte meiosis. Proc Nati Acad Sci U S A. 2013;110:E898-907. doi:10.1073/pnas.1213888110. PMID:23401519
- Cabral G, Sans SS, Cowan CR, Dammermann A. Multiple Mechanisms Contribute to Centriole Separation in C. elegans. Curr Biol. 2013;23:1380-87. doi:10.1016/j.cub.2013.06.043. PMID:23885867
- Mito Y, Sugimoto A, Yamamoto M. Distinct developmental function of two Caenorhabditis elegans homologs of the cohesin subunit Scc1/Rad21. Mol Biol Cell. 2003;14:2399-409. doi:10.1091/mbc.E02-09-0603. PMID:12808038
- Bembenek JN, Richie CT, Squirrell JM, Campbell JM, Eliceiri KW, Poteryaev D, Spang A, Golden A, White JG. Cortical granule exocytosis in C-elegans is regulated by cell cycle components including separase. Development. 2007;134:3837-48. doi:10.1242/dev.011361. PMID:17913784
- Sato M, Grant BD, Harada A, Sato K. Rab11 is required for synchronous secretion of chondroitin proteoglycans after fertilization in Caenorhabditis elegans. J Cell Sci. 2008;121:3177-86. doi:10.1242/jcs.034678. PMID:18765566
- Bembenek JN, White JG, Zheng YX. A Role for Separase in the Regulation of RAB-11-Positive Vesicles at the Cleavage Furrow and Midbody. Curr Biol. 2010;20:259-64. doi:10.1016/j.cub.2009.12.045. PMID:20116245
- Bacac M, Fusco C, Planche A, Santodomingo J, Demaurex N, Leemann-Zakaryan R, Provero P, Stamenkovic I. Securin and Separase Modulate Membrane Traffic by Affecting Endosomal Acidification. Traffic. 2011;12:615-26. doi:10.1111/j.1600-0854.2011.01169.x. PMID:21272169
- Moschou PN, Smertenko AP, Minina EA, Fukada K, Savenkov EI, Robert S, Hussey PJ, Bozhkov PV. The caspase-related protease separase (extra spindle poles) regulates cell polarity and cytokinesis in Arabidopsis (vol 25, pg 2171, 2013). Plant Cell. 2014;26:3823-3823.
- Tsou MF, Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006;442:947-51. doi:10.1038/nature04985. PMID:16862117
- Schockel L, Mockel M, Mayer B, Boos D, Stemmann O. Cleavage of cohesin rings coordinates the separation of centrioles and chromatids. Nat Cell Biol. 2011;13:966-72. doi:10.1038/ncb2280. PMID:21743463
- Peel N, Iyer J, Naik A, Dougherty MP, Decker M, O'Connell KF. Protein Phosphatase 1 Down Regulates ZYG-1 Levels to Limit Centriole Duplication. PLoS Genet. 2017;13, e1006543. doi:10.1371/journal. pgen.1006543. PMID:28103229
- Kachur TM, Audhya A, Pilgrim DB. UNC-45 is required for NMY-2 contractile function in early embryonic polarity establishment and germline cellularization in C-elegans. Dev Biol. 2008;314:287-99. doi:10.1016/j.ydbio.2007.11.028. PMID:18190904
- Bembenek JN, Verbrugghe KJ, Khanikar J, Csankovszki G, Chan RC. Condensin and the spindle midzone prevent cytokinesis failure induced by chromatin bridges in C. elegans embryos. Curr Biol. 2013;23:937-46. doi:10.1016/j.cub.2013.04.028. PMID:23684975
- Norden C, Mendoza M, Dobbelaere J, Kotwaliwale CV, Biggins S, Barral Y. The NoCut pathway links completion of cytokinesis to spindle midzone function to prevent chromosome breakage. Cell. 2006;125:85-98. doi:10.1016/j.cell.2006.01.045. PMID:16615892
- Steigemann P, Wurzenberger C, Schmitz MH, Held M, Guizetti J, Maar S, Gerlich DW. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell. 2009;136:473-84. doi:10.1016/j.cell.2008.12.020. PMID:19203582
- Agromayor M, Martin-Serrano J. Knowing when to but and run: mechanisms that control cytokinetic abscission. Trends Cell Biol. 2013;23:433-41. doi:10.1016/j.tcb.2013.04.006. PMID:23706391
- Jantsch-Plunger V, Glotzer M. Depletion of syntaxins in the early Caenorhabditis elegans embryo reveals a role for membrane fusion events in cytokinesis. Curr Biol. 1999;9:738-45. doi:10.1016/S0960-9822(99)80333-9. PMID:10421575
- Skop AR, Bergmann D, Mohler WA, White JG. Completion of cytokinesis in C-elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr Biol. 2001;11:735-46. doi:10.1016/S0960-9822(01)00231-7. PMID:11378383
- Schiel JA, Childs C, Prekeris R. Endocytic transport and cytokinesis: from regulation of the cytoskeleton to midbody inheritance. Trends Cell Biol. 2013;23:319-27. doi:10.1016/j.tcb.2013.02.003. PMID:23522622
- Albertson R, Riggs B, Sullivan W. Membrane traffic: a driving force in cytokinesis. Trends Cell Biol. 2005;15:92-101. doi:10.1016/j.tcb.2004.12.008. PMID:15695096
- Kimura K, Kimura A. Rab6 is required for the exocytosis of cortical granules and the recruitment of separase to the granules during the oocyte-to-embryo transition in Caenorhabditis elegans. J Cell Sci. 2012;125:5897-905. doi:10.1242/jcs.116400. PMID:22992455
- Olson SK, Greenan G, Desai A, Müller-Reichert T, Oegema K. Hierarchical assembly of the eggshell and permeability barrier in C. elegans. The Journal of Cell Biology. 2012; 198(4):731. doi: 10.1083/jcb.201206008
- Albertson DG, Thomson JN. The Kinetochores of Caenorhabditis-Elegans. Chromosoma. 1982;86;409-28. doi:10.1007/BF00292267. PMID:7172865
- Hehnly H, Chen CT, Powers CM, Liu HL, Doxsey S. The Centrosome Regulates the Rab11-Dependent Recycling Endosome Pathway at Appendages of the Mother Centriole. Curr Biol. 2012;22:1944-50. doi:10.1016/j.cub.2012.08.022. PMID:22981775
- Tang J, Maximov A, Shin OH, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175-87. doi:10.1016/j.cell.2006.08.030. PMID:16990140
- Richie CT, Bembenek JN, Chestnut B, Furuta T, Schumacher JM, Wallenfang M, Golden A. Protein phosphatase 5 is a negative regulator of separase function during cortical granule exocytosis in C. elegans. J Cell Sci. 2011;124:2903-13. doi:10.1242/jcs.073379. PMID:21878498
- Fletcher AI, Shuang RQ, Giovannucci DR, Zhang L, Bittner MA, Stuenkel EL. Regulation of exocytosis by cyclin-dependent kinase 5 via phosphorylation of Munc18. J Biol Chem. 1999;274:4027-35. doi:10.1074/jbc.274.7.4027. PMID:9933594
- Sakaguchi A, Sato M, Sato K, Gengyo-Ando K, Yorimitsu T, Nakai J, Hara T, Sato K, Sato K. REI-1 Is a Guanine Nucleotide Exchange Factor Regulating RAB-11 Localization and Function in C. elegans Embryos. Dev Cell. 2015;35:211-21. doi:10.1016/j.devcel.2015.09.013. PMID:26506309
- Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325-30. doi:10.1038/35042517. PMID:11099033
- Grishok A, Sinskey JL, Sharp PA. Transcriptional silencing of a transgene by RNAi in the soma of C. elegans. Genes Dev. 2005;19:683-96. doi:10.1101/gad.1247705. PMID:15741313
- Edgar LG, Goldstein B. Culture and Manipulation of Embryonic Cells. Caenorhabditis Elegans: Cell Biol Physiology, Second Edition. 2012;07:153-75.
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676-82. doi:10.1038/nmeth.2019. PMID:22743772
- Linkert M, Rueden CT, Allan C, Burel JM, Moore W, Patterson A, Loranger B, Moore J, Neves C, MacDonald D, et al. Metadata matters: access to image data in the real world. J Cell Biol. 2010;189:777-82. doi:10.1083/jcb.201004104. PMID:20513764
