Epithelia are uniformly polarized cell layers held together by specialized adhesion complexes. As the first level of multicellular order to develop during embryonic development, epithelia represent the fundamental building blocks of animal body plans. Throughout development, epithelia undergo continuous remodeling and proliferation but nevertheless maintain integrity through a variety of homeostatic mechanisms. When these mechanisms fail, cancers may arise, as proliferating cells lose control of both cell cycle regulation and epithelial polarity.Citation1 In cells undergoing asymmetric divisions, where daughter cells of different polarity and fate emerge, the cell cycle directly regulates polarity.Citation2 Because mitotic epithelial daughters retain the same polarity after symmetric division, it is assumed that polarized adhesion is stably maintained during the cell cycle. In support, numerous studies in diverse tissues have shown that dividing cells maintain both polarity and adhesion within epithelial layers.Citation3 Here we discuss our recent work on the mitotic control of cell polarity, where we show that apical polarity proteins oscillate in concert with the cell cycle in dividing embryonic cells of the sea anemone Nematostella vectensis.Citation4
As a Cnidarian, Nematostella belongs to an evolutionarily early-branching group of animals that includes sea anemones, corals, and jellyfish- all defined by a simple diploblastic body plan of two epithelial layers which arise through gastrulation of the primary embryonic epithelium. This primary epithelial layer emerges early during Nematostella development, as embryonic cells: 1) Acquire apical polarity by the 4 cell stage; 2) Orient their mitotic spindles parallel to the apical cell surface and 3) Flatten upon each other to form a hollow sphere. Subsequent rapid and synchronous cell divisions increase the cell number of the monolayer without compromising its polarized architecture.Citation4 Interestingly, however, embryos transiently loosen their spherical shape during repeated rounds of synchronous cell division.
Could these dramatic oscillations of embryonic shape be a consequence of temporal loss of epithelial integrity during synchronous cell divisions? To test this possibility, we examined the localization of evolutionarily conserved markers of apical polarity and adhesion (Par-6 and Par-3). Strikingly, shortly after embryonic cells rounded up for mitosis, both Par6-GFP and Par3-GFP intensities were significantly reduced in the apical domain. This reduction lasted only during mitosis and was temporally coupled with apical cell de-adhesion, consistent with a transient disruption of epithelial integrity. Eventually, both Par-6 and Par-3 re-localized to the apical domain as cells re-established their contacts during interphase. Examination of other polarity proteins showed that baso-lateral polarity is unaffected during cell division. Establishing a link with cell cycle regulation, pharmacological inhibition of Cyclin-Dependent Kinases (CDK) blocked apical protein oscillations, indicating that transient loss of apical polarity is either directly or indirectly linked to the cell cycle.Citation4
Our findings in Nematostella complement the observation of cell cycle–coupled epithelial polarity dynamics in diverse Drosophila epithelia.Citation3-6 In Drosophila imaginal discs, for example, baso-lateral junctional localization of the tumor suppressor Lethal Giant Larvae (Lgl) is under cell cycle control. Cell cycle-specific Aurora Kinases phosphorylate Lgl and trigger its release from the cortex into the cytoplasm.Citation5,6 This mechanism, however, does not appear to regulate apical polarity oscillations in Nematostella embryos. Pharmacological inhibition of Aurora Kinases blocked spindle maturation and cytokinesis. Nevertheless, treated embryonic cells still rounded up and apical polarity proteins still oscillated at regular time intervals. We are currently investigating the role of additional cell cycle kinases like Polo-like Kinase 1, which has been shown to regulate internalization of planar cell polarity proteins during mitosis.Citation7 Alternatively, apical polarity oscillations may be attributed to indirect, global cell cycle effects, like changes in acto-myosin-driven cortical flows and / or protein trafficking.
Do cell cycle-coupled apical polarity oscillations play a functional role in developing epithelia? Intriguingly, transient loss of apical protein localization correlated with mitotic cell rounding, while their re-localization to the apical cortex correlated with mitotic exit and cell flattening. As cells flatten they re-establish polarized cell contacts with their neighbors, and the embryo compacts into its spherical interphase shape. These observations suggest that apical protein oscillations may play a role in maintaining epithelial integrity and whole embryo organization. We blocked apical protein recovery at cell contacts by restricting mitotic cells into a rounded shape and examined the epithelial organization of developing embryos. While Par-6 still oscillated at the apical cell surface in synchrony with the cell cycle, it did not stably localize at the rounded cell contacts during interphase, and embryos failed to maintain their monolayer architecture.Citation4 These results indicate that re-establishment of apical adhesions at the completion of cell division is essential for epithelial integrity. The reason why proteins de-localize from the apical domain during cell division remains to be understood.
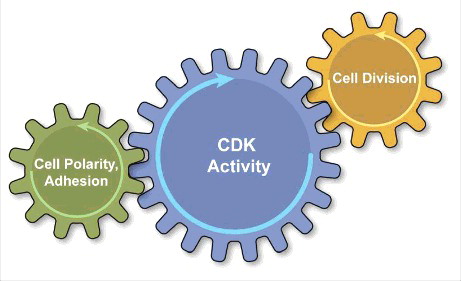
We anticipate future studies will more precisely resolve how the cell cycle machinery regulates polarity protein dynamics in different epithelia. It is possible that these regulatory mechanisms will vary significantly between different tissues and organisms, but as a general theme we envision a model where oscillations in polarized adhesion and the nuclear events of cell division are ultimately coordinated in unison by a centralized CDK program ().
Figure 1. The epithelial cell cycle depicted as a combination of clocks, with the central clock (blue-CDK activity) tuning the cycling of independent cell processes presented as peripheral clocks. The centralized CDK program coordinates the oscillations in nuclear and cytoplasmic events of cell division (yellow) with the oscillations in cortical cell polarity and inter-cellular adhesion (green).
References
- Muthuswamy SK, Xue B. Cell polarity as a regulator of cancer cell behavior plasticity. Annu Rev Cell Dev Biol. 2012;28:599–625. doi:10.1146/annurev-cellbio-092910-154244. PMID:22881459
- Noatynska A, Tavernier N, Gotta M, Pintard L. Coordinating cell polarity and cell cycle progression: what can we learn from flies and worms? Open Biol. 2013;3(8):130083. doi:10.1098/rsob.130083. PMID:23926048
- Le Bras S, Le Borgne R. Epithelial cell division – multiplying without losing touch. J Cell Sci. 2014;127(Pt 24):5127–37. doi:10.1242/jcs.151472. PMID:25344250
- Ragkousi K, Marr K, McKinney S, Ellington L, Gibson MC. Cell-cycle-coupled oscillations in apical polarity and intercellular contact maintain order in embryonic epithelia. Curr Biol. 2017;27(9):1381–1386. doi:10.1016/j.cub.2017.03.064. PMID:28457868
- Carvalho CA, Moreira S, Ventura G, Sunkel CE, Morais-de-Sá E. Aurora A triggers Lgl cortical release during symmetric division to control planar spindle orientation. Curr Biol. 2015;25(1):53–60. doi:10.1016/j.cub.2014.10.053. PMID:25484294
- Bell GP, Fletcher GC, Brain R, Thompson BJ. Aurora kinases phosphorylate Lgl to induce mitotic spindle orientation in Drosophila epithelia. Curr Biol. 2015;25(1):61–8. doi:10.1016/j.cub.2014.10.052. PMID:25484300
- Shrestha R, Little KA, Tamayo JV, Li W, Perlman DH, Devenport D. Mitotic control of planar cell polarity by Polo-like Kinase 1. Dev Cell. 2015;33(5):522–34. doi:10.1016/j.devcel.2015.03.024. PMID:26004507
