ABSTRACT
The elevated methionine (MET) use by cancer cells is termed MET dependence and may be the only known general metabolic defect in cancer. Targeting MET by recombinant methioninase (rMETase) can arrest the growth of cancer cells in vitro and in vivo. We previously reported that rMETase, administrated by intra-peritoneal injection (ip-rMETase), could inhibit tumor growth in a patient-derived orthotopic xenograft (PDOX) model of a BRAF-V600E mutant melanoma. In the present study, we compared ip-rMETase and oral rMETase (o-rMETase) for efficacy on the melanoma PDOX. Melanoma PDOX nude mice were randomized into four groups of 5 mice each: untreated control; ip-rMETase (100 units, i.p., 14 consecutive days); o-rMETase (100 units, p.o., 14 consecutive days); o-rMETase+ip-rMETase (100 units, p.o.+100 units, i.p., 14 consecutive days). All treatments inhibited tumor growth on day 14 after treatment initiation, compared to untreated control (ip-rMETase, p<0.0001; o-rMETase, p<0.0001; o-rMETase+ip-rMETase, p<0.0001). o-rMETase was significantly more effective than ip-rMETase (p = 0.0086). o-rMETase+ip-rMETase was significantly more effective than either mono-therapy: ip-rMETase, p = 0.0005; or o-rMETase, p = 0.0367. The present study is the first demonstrating that o-rMETase is effective as an anticancer agent. The results of the present study indicate the potential of clinical development of o-rMETase as an agent for chronic cancer therapy and for cancer prevention and possibly for life extension since dietary MET reduction extends life span in many animal models.
Introduction
Metastatic melanoma is a recalcitrant cancer, with a 5-y survival rate of 7–30% with no cure for stage III and IV melanoma [Citation1–5]. Vitamin D and its receptors play a critical role in signaling in melanoma progression [Citation6].
An excessive requirement for methionine, termed methionine dependence, appears to be a general metabolic defect in cancer [Citation7]. We have previously shown that cancer-cell growth can be selectively arrested by methionine deprivation, such as with recombinant methioninase (rMETase) [Citation7].
In previous studies, we established patient-derived orthotopic xenograft (PDOX) nude mouse model of BRAF V600E-mutant melanoma [Citation8–Citation12]. In this model, we determine the efficacy of rMETase in combination with a first-line melanoma drug, temozolomide (TEM) [Citation11]. The combination therapy of TEM, first-line therapy, and rMETase was significantly more efficacious than either mono-therapy [Citation11].
The present study demonstrates that oral rMETase (o-rMETase) is highly effective in the BRAF-V600E mutant melanoma PDOX.
Results and discussion
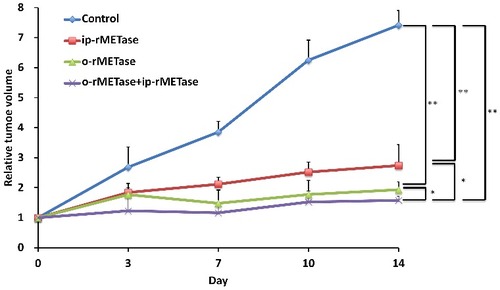
Melanoma-PDOX nude mice were randomized into four groups of 5 mice each: untreated control; ip-rMETase (100 units, i.p., 14 consecutive days); o-rMETase (100 units, p.o., 14 consecutive days); o-rMETase+ip-rMETase (100 units, p.o.+100 units, i.p., 14 consecutive days). All treatments inhibited tumor growth on d 14 after treatment initiation, compared to untreated control (ip-rMETase, p<0.0001; o-rMETase, p<0.0001; o-rMETase+ip-rMETase, p<0.0001). o-rMETase was significantly more effective than ip-rMETase (p = 0.0086). The combination of o-rMETase+ip-rMETase was significantly more effective than either monotherapy therapies: ip-rMETase, p = 0.0005; or o-rMETase, p = 0.0367 ( and ).
Figure 1. Photographs of representative tumors from the untreated control and treatment groups on the BRAF V600E mutant-melanoma PDOX. Tumors were resected on d 15 of treatment. Scale bar: 5 mm
Figure 2. Quantitative efficacy of ip-rMETase, o-rMETase and the combination of ip-rMETase and o-rMETase on the BRAF V600E mutant melanoma PDOX. Line graphs show relative tumor volume at each point relative to the initial tumor volume. ##p < 0.01, #p < 0.05. Error bars: ± SD.
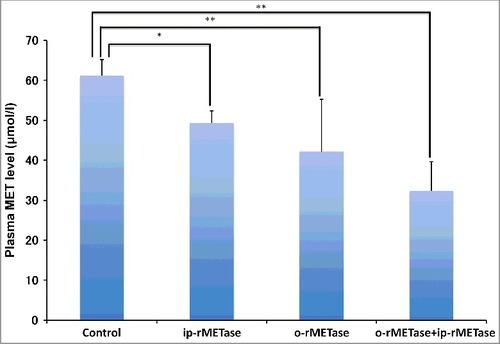
Post-treatment plasma MET levels significantly decreased compared to untreated control: ip-rMETase, p = 0.0122; o-rMETase, p = 0.003; o-rMETase+ip-rMETase, p<0.0001 ().
Figure 3. Effect of ip-rMETase and o-rMETase on plasma rMETase levels. Bar graphs show plasma MET levels in each group at post-treatment. ##p < 0.01, #p < 0.05. Error bars: ± SD.
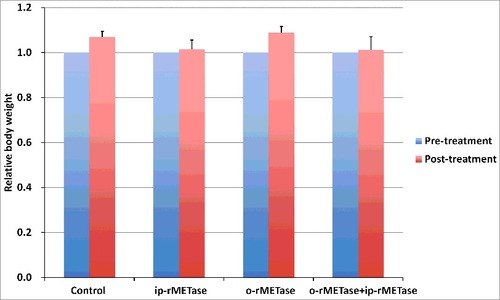
Body weight loss was not observed in any treatment group (). There were no animal deaths in any group.
These results showed the safety of o-rMETase and its potential for chronic cancer treatment in the clinic.
Figure 4. Effect of ip-rMETase, o-rMETase and the combination of ip-rMETase and o-rMETase on the BRAF V600E mutant-melanoma PDOX mouse body weight. Bar graphs show mouse body weight in each treatment group at pre- and post-treatment. There were no significantly differences between any treatment group and untreated control.
MET dependence is a general metabolic defect in cancer. Methionine dependence is due to excess use of MET for aberrant transmethylation reactions, termed the “Hoffman effect”, analogous to the Warburg effect for elevated glucose use in cancer [Citation7,Citation13–18]. The excessive and aberrant use of MET in cancer is readily observed in [11C]MET PET imaging, where high uptake of [11C]MET results in a very strong and selective tumor signal compared with normal tissue background. [11C]MET is superior to [18C] fluorodeoxyglucose (FDG) for PET imaging, suggesting MET dependence is more tumor-specific than glucose dependence [Citation19,Citation20].
MET is sourced mainly from food. However, MET restriction through diets with low protein content does not allow the maintenance of good nutritional status. In addition, reduction of MET levels by dietary intervention is limited since MET is sourced from protein breakdown [Citation7]. Further reduction of plasma MET, and thereby tumor MET, has been achieved with the use of rMETase [Citation11,Citation21–25].
Our laboratory cloned Pseudomonas putida rMeTase into E. coli for large scale production [Citation26]. It has been demonstrated that MET deprivation arrests growth and induces a tumor-selective G2-phase cell-cycle arrest of cancer cells in vitro and in vivo [Citation27–30].
We reported recently on the efficacy of rMETase against Ewing's sarcoma in a PDOX model. rMETase effectively reduced tumor growth compared to untreated control. Serum and tumor met levels were lower in the rMETase group [Citation25].
In a previous study, the BRAF V600E-mutant melanoma PDOX was sensitive to rMETase and increased TEM efficacy in combination [Citation11].
The present study reports the surprising result that o-rMETase is effective against the BRAF-V600E mutant melanoma PDOX and is more effective than ip-rMETase. The use of o-rMETase opens many possibilities that appear non-toxic for chronic cancer treatment, for cancer prevention and for general life span extension since MET is also a target of aging [Citation31,Citation32]. A future new study will focus on effects of oral methioninase on tumor histology.
The present study demonstrates the power of the PDOX model to identify effective therapy for recalcitrant cancer. Toward this goal of precision personalized oncology, our laboratory pioneered the patient-derived orthotopic xenograft (PDOX) nude mouse model with the technique of surgical orthotopic implantation (SOI), including pancreatic [Citation33–Citation36], breast [Citation37], ovarian [Citation38], lung [Citation39], cervical [Citation40], colon [Citation41–43] and stomach cancer [Citation44], sarcoma [Citation45–49] and melanoma [Citation8–11,Citation50].
Previously-developed concepts and strategies of highly-selective tumor targeting can take advantage of molecular targeting of tumors, including tissue-selective therapy which focuses on unique differences between normal and tumor tissues [Citation51–56].
Materials and methods
Mice
Athymic nu/nu nude mice (AntiCancer Inc., San Diego, CA), 4–6 wk old, were used in this study. Mice were housed in a barrier facility in a high efficacy particulate arrestance (HEPA)-filtered rack under standard conditions of 12-hour light/dark cycles. The animals were fed an autoclaved laboratory rodent diet. All animal studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals under Assurance Number A3873-1. All mouse surgical procedures and imaging were performed with the animals anesthetized by subcutaneous injection of a ketamine mixture (0.02 ml solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate). The response of animals during surgery was monitored to ensure adequate depth of anesthesia. The animals were observed on a daily basis and humanely sacrificed by CO2 inhalation if they met the following endpoint criteria: severe tumor burden (more than 20 mm in diameter), prostration, significant body-weight loss, difficulty breathing, rotational motion or body temperature drop [Citation11].
Patient-derived tumor
A 75-y old female patient was previously diagnosed with a BRAF-V600E melanoma of the right chest wall. The tumor was previously resected in the Department of Surgery, University of California, Los Angeles (UCLA). Written informed consent was provided by the patient, and the Institutional Review Board (IRB) of UCLA approved this experiment [Citation8–11].
Establishment of PDOX models of melanoma by surgical orthotopic implantation (SOI)
Subcutaneously-grown BRAF V600E mutant melanoma was harvested and cut into small fragments (3 mm3). After nude mice were anesthetized with the ketamine solution described above, a 5-mm skin incision was made on the right chest into the chest wall, which was split to make space for the melanoma tissue fragment. A single tumor fragment was implanted orthotopically into the space to establish the PDOX model. The wound was closed with a 6-0 nylon suture (Ethilon, Ethicon, Inc., NJ, USA) [Citation8–11].
Recombinant methionase (rMETase) production
Recombinant L-metionine α-deamino-γ-mercaptomethane lyase (recombinant methioninase [rMETase]) [EC 4.4.1.11] from Pseudomonas putida has been previously cloned and was produced in Escherichia coli (AntiCancer, Inc., San Diego, CA). rMETase is a homotetrameric PLP enzyme of 172-kDa molecular mass [Citation26].
Formulation of o-rMETase and pyridoxal-L-phosphate (PLP) supplement
Mouse drinking water contained 100 µmol/l PLP. PLP (1.0 ml of 20 mmol/l) was added to 200 ml drinking water and made fresh daily. rMETase was administered daily by gavage using a stainless feeding needle (100 units, 2.0 mg) in phosphate-buffered saline (PBS).
Treatment study design in the PDOX model of melanoma
BRAF V600E mutant melanoma PDOX nude mice were randomized into four groups of 5 mice each: untreated control; ip-rMETase (100 units, i.p., 14 consecutive days); o-rMETase (100 units, p.o., 14 consecutive days); o-rMETase+ip-rMETase (100 units, p.o.+100 units, i.p., 14 consecutive days).
Determination of Plasma methionine
The plasma methionine concentration was measured using a precolumn derivatization, followed by high-performance liquid chromatography separation based on a previously described method with modification [Citation57]. A 10-μl plasma sample or methionine standard was used. The plasma methionine was identified by the retention time of a methionine standard curve. The limit of detection was 0.5 μM methionine. The upper limit of detection for methionine for methionine assay is 100μM.
Statistical analysis
JMP version 11.0 was used for all statistical analyses. Significant differences for continuous variables were determined using the Mann-Whitney U test. Line graphs express average values and error bar show SD. A probability value of P ≤ 0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi:10.1056/NEJMoa1103782. PMID:21639808
- Flaherty LE, Othus M, Atkins MB, et al. Southwest Oncology Group S0008: a phase III trial of high-dose interferon Alfa-2b versus cisplatin, vinblastine, and dacarbazine, plus interleukin-2 and interferon in patients with high-risk melanoma–an intergroup study of cancer and leukemia Group B, Children's Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. J Clin Oncol. 2014;32:3771–3778. doi:10.1200/JCO.2013.53.1590. PMID:25332243
- Tang H, Wang Y, Chlewicki LK, et al. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell. 2016;29:285–296. doi:10.1016/j.ccell.2016.02.004. PMID:26977880
- Brozyna AA, Jóźwicki W, Roszkowski K, et al. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget. 2016;7:17844–17853. doi:10.18632/oncotarget.7528. PMID:26910282
- Slominski AT, Carlson JA. Melanoma resistance: a bright future for academicians and a challenge for patient advocates. Mayo Clin Proc. 2014;89:429–433. doi:10.1016/j.mayocp.2014.02.009. PMID:24684870
- Slominski AT, Brożyna AA, Zmijewski MA, et al. Vitamin D signaling and melanoma: role of vitamin D and its receptors in melanoma progression and management. Lab Invest. 2017;97:706–724. doi:10.1038/labinvest.2017.3. PMID:24684870
- Hoffman RM. Development of recombinant methioninase to target the general cancer-specific metabolic defect of methionine dependence: a 40-year odyssey. Expert Opin Biol Ther. 2015;15:21–31. doi:10.1517/14712598.2015.963050. PMID:25439528
- Kawaguchi K, Murakami T, Chmielowski B, et al. Vemurafenib-resistant BRAF-V600E mutated melanoma is regressed by MEK targeting drug trametinib, but not cobimetinib in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget. 2016;7:71737–71743. PMID:27690220
- Kawaguchi K, Igarashi K, Murakami T, et al. Tumor-targeting Salmonella typhimurium A1-R combined with temozolomide regresses malignant melanoma with a BRAF-V600 mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget. 2016;7:85929–85936.
- Kawaguchi K, Igarashi K, Murakami T, et al. Tumor-targeting Salmonella typhimurium A1-R sensitizes melanoma with a BRAF-V600E mutation to vemurafenib in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Cell Biochem. 2017;118:2314–2319. doi:10.1002/jcb.25886. PMID:PMID:28106277 PMID:27835903.
- Kawaguchi K, Igarashi K, Li S, et al. Combination treatment with recombinant methioninase enables temozolomide to arrest a BRAF V600E melanoma growth in a patient-derived orthotopic xenograft. Oncotarget. 2017;8:85516–85525. PMID:29156737
- Hoffman RM. Patient-derived orthotopic xenografts (PDOX) models of melanoma. Special Issue “Animal Models of Melanoma”, Slominski, A., Guest Editor. Intl J Mol Sci. 2017;18:1875. doi:10.3390/ijms18091875.
- Hoffman RM, Erbe RW. High in vivo rates of methionine biosynthesis in transformed human and malignant rat cells auxotrophic for methionine. Proc Natl Acad Sci U S A. 1976;73:1523–1527. doi:10.1073/pnas.73.5.1523. PMID:179090
- Stern PH, Mecham JO, Wallace CD, et al. Reduced free-methionine in methionine-dependent SV40-transformed human fibroblasts synthesizing apparently normal amounts of methionine. J Cell Physiol. 1983;117:9–14. doi:10.1002/jcp.1041170103. PMID:6311851
- Stern PH, Wallace CD, Hoffman RM. Altered methionine metabolism occurs in all members of a set of diverse human tumor cell lines. J Cell Physiol. 1984;119:29–34. doi:10.1002/jcp.1041190106. PMID:6707100
- Stern PH, Hoffman RM. Elevated overall rates of transmethylation in cell lines from diverse human tumors. In Vitro. 1984;20:663–670. doi:10.1007/BF02619617. PMID:6500606
- Hoffman RM. Altered methionine metabolism, DNA methylation and oncogene expression in carcinogenesis: a review and synthesis. Biochim Biophys Acta. 1984;738:49–87. PMID:6204687
- Coalson DW, Mecham JO, Stern PH, et al. Reduced availability of endogenously synthesized methionine for S-adenosylmethionine formation in methionine dependent cancer cells. Proc Natl Acad Sci U S A. 1982;79:4248–4251. doi:10.1073/pnas.79.14.4248. PMID:6289297
- Hoffman RM. Is DNA methylation the new guardian of genome? Mol Cytogenetics. 2017;10:11. doi:10.1186/s13039-017-0314-8.
- Hoffman RM. The wayward methyl group and the cascade to cancer. Cell Cycle. 2017;16:825–829. doi:10.1080/15384101.2017.1304330. PMID:28318368
- Tan Y, Sun X, Xu M, et al. Efficacy of recombinant methioninase in combination with cisplatin on human colon tumors in nude mice. Clin Cancer Res. 1999;5:2157–2163. PMID:10473100
- Tan Y, Zavala J Sr, Xu M, et al. Serum methionine depletion without side effects by methioninase in metastatic breast cancer patients. Anticancer Res. 1996;16:3937–3942. PMID:9042316
- Tan Y, Zavala J Sr, Han Q, et al. Recombinant methioninase infusion reduces the biochemical endpoint of serum methionine with minimal toxicity in high-stage cancer patients. Anticancer Res. 1997;17:3857–3860. PMID:9427792
- Kokkinakis DM, Hoffman RM, Frenkel EP, et al. Synergy between methionine stress and chemotherapy in the treatment of brain tumor xenografts in athymic mice. Cancer Res. 2001;61:4017–4023. PMID:11358820
- Murakami T, Li S, Han Q, et al. Recombinant methioninase effectively targets a Ewing's sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2017;8:35630–35638. PMID:28404944
- Tan Y, Xu M, Tan X, et al. Overexpression and large-scale production of recombinant L-methionine-alpha- deamino-gamma-mercaptomethane-lyase for novel anticancer therapy. Protein Expr Purif. 1997;9:233–245. doi:10.1006/prep.1996.0700. PMID:9056489
- Guo H, Lishko VK, Herrera H, et al. Therapeutic tumor-specific cell cycle block induced by methionine starvation in vivo. Cancer Res. 1993;53:5676–5679 PMID:8242623
- Hoffman RM, Jacobsen SJ. Reversible growth arrest in simian virus 40-transformed human fibroblasts. Proc Natl Acad Sci U S A. 1980;77:7306–7310. doi:10.1073/pnas.77.12.7306. PMID:6261250
- Kokkinakis DM, von Wronski MA, Vuong TH, et al. Regulation of O6-methylguanine-DNA methyltransferase by methionine in human tumour cells. Br J Cancer. 1997;75:779–788. doi:10.1038/bjc.1997.141. PMID:9062396
- Kokkinakis DM, Schold SC Jr, Hori H, et al. Effect of long-term depletion of plasma methionine on the growth and survival of human brain tumor xenografts in athymic mice. Nutr Cancer. 1997;29:195–204. doi:10.1080/01635589709514624. PMID:9457739
- Lishko VK, Lishko OV, Hoffman RM. The preparation of endotoxin-free L-methionine-alpha-deamino-gamma-mercaptomethane-lyase (L-methioninase) from Pseudomonas putida. Protein Expr Purif. 1993;4:529–533. doi:10.1006/prep.1993.1069. PMID:8286949
- Tan Y, Zavala J Sr, Xu M, et al. Serum methionine depletion without side effects by methioninase in metastatic breast cancer patients. Anticancer Res. 1996;16:3937–3942. PMID:9042316
- Hiroshima Y, Zhang Y, Murakami T, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograph (PDOX) and cell line mouse models. Oncotarget. 2014;5:12346–12357. doi:10.18632/oncotarget.2641. PMID:25402324
- Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci U S A. 1992;89:5645–5649. doi:10.1073/pnas.89.12.5645. PMID:1608975
- Hiroshima Y, Maawy A, Zhang Y, et al. Metastatic recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) nude mouse model is inhibited by neoadjuvant chemotherapy in combination with fluorescence-guided surgery with an anti-CA 19–9-conjugated fluorophore. PLoS One. 2014;9:e114310. doi:10.1371/journal.pone.0114310. PMID:25463150
- Hiroshima Y, Maawy AA, Katz MH, et al. Selective efficacy of zoledronic acid on metastasis in a patient-derived orthotopic xenograph (PDOX) nude-mouse model of human pancreatic cancer. J Surg Oncol. 2015;111:311–315. doi:10.1002/jso.23816. PMID:25394368
- Fu X, Le P, Hoffma RM. A metastatic-orthotopic transplant nude-mouse model of human patient breast cancer. Anticancer Res. 1993;13:901–904. PMID:8352558
- Fu X, Hoffman RM. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res. 1993;13:283–286. PMID:8517640
- Wang X, Fu X, Hoffman RM. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int J Cancer. 1992;51:992–995. doi:10.1002/ijc.2910510626. PMID:1639545
- Hiroshima Y, Zhang Y, Zhang N, et al. Establishment of a patient-derived orthotopic xenograph (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLoS One. 2015;10:e0117417. doi:10.1371/journal.pone.0117417. PMID:25689852
- Fu X, Besterman JM, Monosov A, et al. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci U S A. 1991;88:9345–9349. doi:10.1073/pnas.88.20.9345. PMID:1924398
- Metildi CA, Kaushal S, Luiken GA, et al. Fluorescently-labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Surg Oncol. 2014;109:451–458. doi:10.1002/jso.23507. PMID:24249594
- Hiroshima Y, Maawy A, Metildi CA, et al. Successful fluorescence-guided surgery on human colon cancer patient-derived orthotopic xenograft mouse models using a fluorophore-conjugated anti-CEA antibody and a portable imaging system. J Laparoendosc Adv Surg Tech A. 2014;24:241–247. doi:10.1089/lap.2013.0418. PMID:24494971
- Furukawa T, Kubota T, Watanabe M, et al. Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: correlation of metastatic sites in mouse and individual patient donors. Int J Cancer. 1993;53:608–612. doi:10.1002/ijc.2910530414. PMID:8436434
- Murakami T, DeLong J, Eilber FC, et al. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft PDOX model. Oncotarget. 2016;7:12783–12790. doi:10.18632/oncotarget.7226. PMID:26859573
- Hiroshima Y, Zhao M, Zhang Y, et al. Tumor-targeting Salmonella typhimurium A1-R arrests a chemo-resistant patient soft-tissue sarcoma in nude mice. PLoS One. 2015;10:e0134324. doi:10.1371/journal.pone.0134324. PMID:26237416
- Kiyuna T, Murakami T, Tome Y, et al. High efficacy of tumor-targeting Salmonella typhimurium A1-R on a doxorubicin- and dactolisib-resistant follicular dendritic-cell sarcoma in a patient-derived orthotopic xenograft PDOX nude mouse model. Oncotarget. 2016;7:33046–33054. doi:10.18632/oncotarget.8848. PMID:27105519
- Murakami T, Singh AS, Kiyuna T, et al. Effective molecular targeting of CDK4/6 and IGF-1R in a rare FUS-ERG fusion CDKN2A-deletion doxorubicin-resistant Ewing's sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2016;7:47556–47564. doi:10.18632/oncotarget.9879. PMID:27286459
- Hiroshima Y, Zhang Y, Zhang N, et al. Patient-derived orthotopic xenograft (PDOX) nude mouse model of soft-tissue sarcoma more closely mimics the patient behavior in contrast to the subcutaneous ectopic model. Anticancer Res. 2015;35:697–701. PMID:25667448
- Yamamoto M, Zhao M, Hiroshima Y, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R on a melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. PLoS One. 2016;11:e0160882. doi:10.1371/journal.pone.0160882. PMID:27500926
- Blagosklonny MV. Matching targets for selective cancer therapy. Drug Discov Today. 2003;8:1104–1107. doi:10.1016/S1359-6446(03)02806-X. PMID:14678733
- Blagosklonny MV. Teratogens as anti-cancer drugs. Cell Cycle. 2005;4:1518–1521. doi:10.4161/cc.4.11.2208. PMID:16258270
- Blagosklonny MV. Treatment with inhibitors of caspases, that are substrates of drug transporters, selectively permits chemotherapy-induced apoptosis in multidrug-resistant cells but protects normal cells. Leukemia. 2001;15:936–941. doi:10.1038/sj.leu.2402127. PMID:11417480
- Blagosklonny MV. Target for cancer therapy: proliferating cells or stem cells. Leukemia. 2006;20:385–391. doi:10.1038/sj.leu.2404075. PMID:16357832
- Apontes P, Leontieva OV, Demidenko ZN, et al. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget. 2011;2:222–233. doi:10.18632/oncotarget.248. PMID:21447859
- Blagosklonny MV. Tissue-selective therapy of cancer. Br J Cancer. 2003;89:1147–1151. doi:10.1038/sj.bjc.6601256. PMID:14520435
- Jones BN, Gilligan JP. o-Phthaldialdehyde precolumn derivatization and reversed-phase high-performance liquid chromatography of polypeptide hydrolysates and physiological fluids. J Chromatogr. 1983;266:471–482. doi:10.1016/S0021-9673(01)90918-5. PMID:6630358
