ABSTRACT
Paeoniflorin (PF) exhibits tumor suppressive functions in a variety of human cancers. However, the function of PF and molecular mechanism in colorectal cancer are elusive. In the present study, we investigated whether PF could exert its antiproliferative activity, anti-migration, and anti-invasive function in colorectal cancer cells. We found that PF inhibited cell growth and induced apoptosis and blocked cell cycle progression in the G0/G1 phase in colorectal cancer cells. Moreover, we found that PF suppressed cell migration and invasion in colorectal cancer cells. FoxM1 has been reported to play an important oncogenic role in human cancers. We also determine whether PF inhibited the expression of FoxM1, leading to its anti-cancer activity. We found that PF treatment in colorectal cancer cells resulted in down-regulation of FoxM1. The rescue experiments showed that overexpression of FoxM1 abrogated the tumor suppressive function induced by PF treatment. Notably, depletion of FoxM1 promoted the anti-tumor activity of PF in colorectal cancer cells. Therefore, inhibition of FoxM1 could participate in the anti-tumor activity of PF in colorectal cancer cells.
Introduction
Colorectal cancer is one of the most commonly diagnosed cancers, which remains the second leading cause of cancer-related deaths in the United States [Citation1]. Due to improved diagnosis, systemic therapies and ablative techniques, the clinical outcome of colorectal cancer patients has been improved [Citation2]. It has been reported that colorectal cancer is increasing in young adults partly due to increasing initiation of screen. However, a median overall survival of metastatic colorectal cancer patients is approximately 24–30 months, suggesting that new therapeutic approaches are required to develop for the treatment of patients with colorectal cancer [Citation3].
FoxM1, a member of the Fox transcription factor family, has been initially report to control cell proliferation and apoptosis [Citation4,Citation5]. It has been known that FoxM1 governs multiple cellular progressions including development, differentiation, migration, invasion, and metastasis [Citation6,Citation7]. For example, FoxM1 regulates cell cycle progression via targeting several cell cycle regulators including Cdc25A, Cdc25B, cyclin B, cyclin D1, p21cip1 and p27kip1 [Citation8,Citation9]. Recent studies identified that dysfunction of FoxM1 is associated with tumorigenesis. Over-expression of FoxM1 has been reported in a variety of human cancers including lung cancer, prostate cancer, hepatocellular carcinoma, breast cancer and pancreatic cancer [Citation10–13]. These findings reveled that FoxM1 plays an oncogenic role in tumorigenesis [Citation14]. Therefore, targeting FoxM1 could be a promising approach for the treatment of human cancers.
Paeoniflorin (PF), the principal bioactive component that isolated from the paeony root, has been demonstrated to exhibit several biological functions, such as immunoregulatory, anticonvulsant, anti hyperglycaemic and antihypotensive effects [Citation15,Citation16]. Recent studies indicate that PF possesses its anti-tumor activity in various types of human cancers [Citation17–20]. However, the function of PF and molecular mechanism in colorectal cancer are largely unclear. In the present study, we investigated whether PF could exert its anti-tumor activity in colorectal cancer cells. We found that PF inhibited cell growth and induced apoptosis and blocked cell cycle progression. Moreover, PF retarded cell migration and invasion in colorectal cancer cells. We also determine whether PF could inhibit the expression of FoxM1 in colorectal cancer cells. We found that FoxM1 was significantly reduced in PF-treated cells. Moreover, overexpression of FoxM1 abrogated the tumor suppressive function induced by PF treatment. Furthermore, depletion of FoxM1 promoted the anti-tumor activity of PF in colorectal cancer cells. Therefore, PF could be a useful agent to inhibit FoxM1 expression for the treatment of colorectal cancer patients.
Results
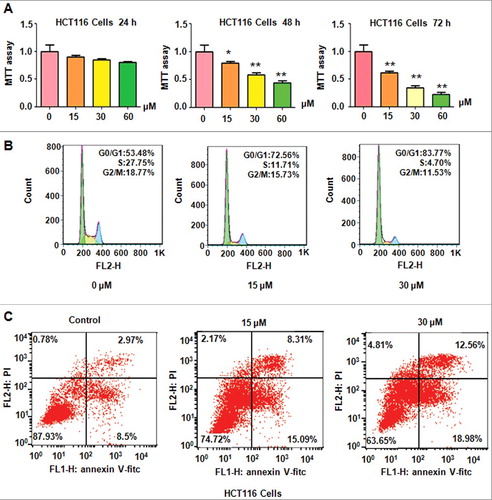
PF inhibited colon cancer cell growth
PF has been reported to inhibit cell growth in several types of human cancer cell lines [Citation19,Citation21]. We investigated whether PF could inhibit colon cancer cell growth. HCT116 cells were treated with 15 μM, 30 μM, and 60 μM PF for 24 h, 48 h, and 72 h, respectively. We found that PF suppressed cell growth in a time- and dose-dependend manner in HCT116 cells (A). Briefly, 30 μM and 60 μM PF treatments for 48 hours resulted in about 40% and 60% of cell growth inhibition in HCT116 cells (A). Moreover, 30 μM PF treatments for 72 hours led to 60% of cell growth suppression (A). Our MTT results clear suggested that PF suppressed colon cancer cell growth.
PF induced cell cycle arrest in colon cancer cells
Next, we performed cell cycle analysis using PI staining and flow cytometry in HCT116 cells with PF treatments. We observed that PF treatment induced cell cycle arrest at G0/G1 phase in HCT116 cells (B). Specifically, 15 μM and 30 μM PF treatment led to G0/G1 phase from 53.48% in control group to 72.56% and 83.77% in HCT116 cells, respectively (B). Our data demonstrated that PF induced cell cycle at G0/G1 phase in colon cancer cells.
PF induced cell apoptosis in colon cancer cells
We also explored whether PF could induce cell apoptosis in colon cancer cells. Annexin V-FITC/PI assay was conducted to analyze cell apoptosis in HCT116 cells after PF treatments with different concentrations of PF for 48 h. we found that PF induced cell apoptosis in HCT116 cells (C). Specifically, 15 μM and 30 μM PF treatments induced cell apoptotic death from 11.4% of control group to 23.4% and 31.5% in HCT116 cells (C). Therefore, PF treatments triggered cell apoptosis in colon cancer cells.
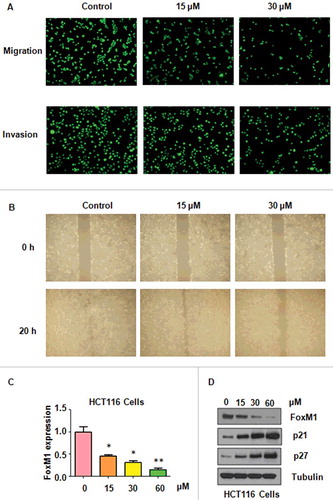
PF inhibited cell migration and invasion in colon cancer cells
To further dissect whether PF could inhibit colon cancer cell motility, Transwel assay was performed in HCT116 cells with PF treatments. We found that PF significantly inhibited cell migration in HCT116 cells (A). Moreover, our invasion assay results demonstrated that cell invasion was inhibited by PF treatment in HCT116 cells (A). Furthermore, our wound healing assay results showed that cell motility was remarkably suppressed by PF treatment in HCT116 cells (B). Our data suggested that PF treatments retarded cell migration and invasion in colon cancer cells.
PF inhibited FoxM1 expression in colon cancer cells
FoxM1 has been reported to play an oncogenic role in colon cancer [Citation22,Citation23]. We investigated whether FoxM1 could be inhibited by PF in colon cancer cells. Real-time RT-PCR and Western blotting were performed to measure the mRNA and protein levels of FoxM1 in colon cancer cells after PF treatments. We found that PF reduced FoxM1 mRNA level in HCT116 cells (C). Our Western blotting results showed that FoxM1 expression was decreased in HCT116 cells after PF treatment (D). In line with this, we found that PF treatment increased the expression of p21 and p27, two downstream targets of FoxM1, in colon cancer cells (D). Thus, PF exhibited its anti-cancer activity partly due to inhibition of FoxM1 in colon cancer cells.
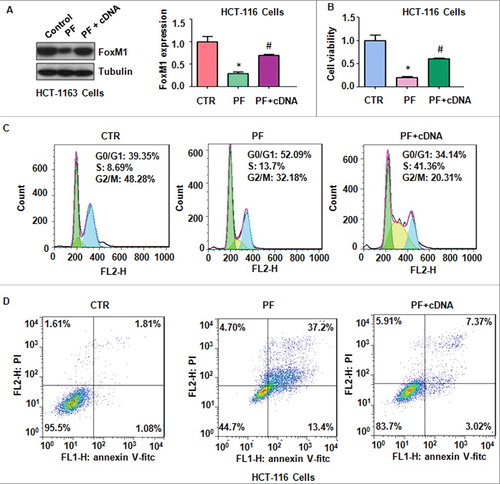
Over-expression of FoxM1 rescued PF-induced cell growth inhibition and apoptosis
To further confirm whether PF exerted its anti-tumor activity through suppression of FoxM1 in colon cancer cells, HCT116 cells were transfected with FoxM1 cDNA or pcDNA 3.1 as control group. We observed that FoxM1 transfection rescued the inhibition of FoxM1 induced by PF treatment in HCT116 cells (A). Our MTT results showed that up-regulation of FoxM1 abrogated PF-induced cell growth suppression in HCT116 cells (B). We also found that over-expression of FoxM1 rescued PF-trigged cell cycle arrest at G0/G1 phase (C). Notably, over-expression of FoxM1 reduced PF-mediated cell apoptosis in HCT116 cells (D).
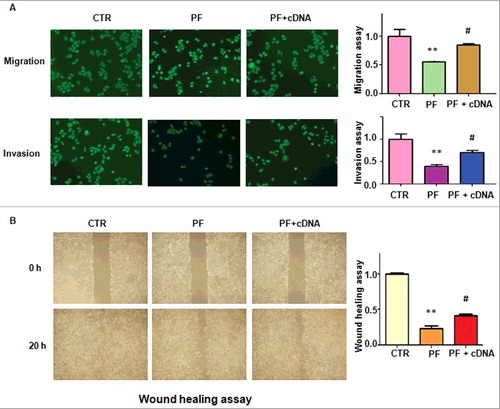
Over-expression of FoxM1 abolished PF-induced cell motility inhibition.
To determine whether PF retarded cell migration and invasion via down-regulation of FoxM1 in colon cancer cells, Transwell assay was used to measure cell motility in HCT116 cells after PF treatment. We found that overexpression of FoxM1 abrogated the inhibition of cell migration and invasion induced by PF in HCT116 cells (A). Our wound healing assay further confirmed that up-regulation of FoxM1 rescued the inhibitory effects of PF on cell invasive activity (B). These results indicated that PF exerts its anti-motility function in part via inhibition of FoxM1 in colon cancer cells.
Depletion of FoxM1 promoted PF-induced anti-tumor activities
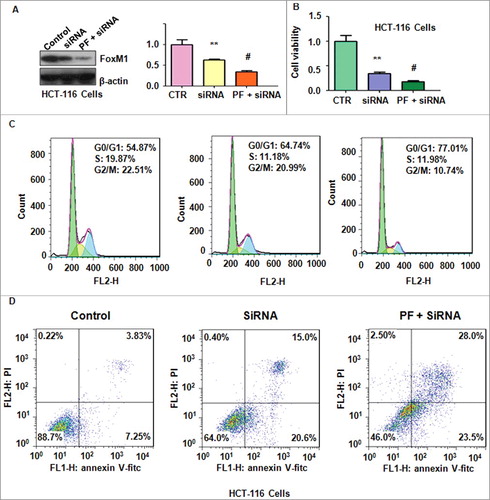
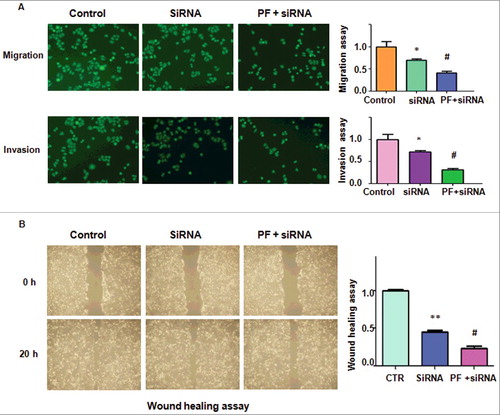
To further validate whether PF exerted its tumor suppressive function via FoxM1 signaling pathway, FoxM1 siRNA oligonucleotides was used in colon cancer cells after PF treatment. We found that FoxM1 expression was decreased after its siRNA transfection in HCT116 cells (A). FoxM1 siRNA transfection in combination with PF treatment led to less expression of FoxM1 compared with siRNA treatment alone (A). Our MTT results showed that depletion of FoxM1 plus PF treatment promoted cell growth inhibition to a greater degree compared with siRNA treatment alone (B). Furthermore, down-regulation of FoxM1 enhanced PF-induced G0/G1 cell cycle arrest (C). Strikingly, depletion of FoxM1 enhanced PF-mediated cell apoptosis in HCT116 cells (D). Additionally, FoxM1 siRNA transfection in combination with PF treatment led to inhibition of cell migration and invasion to a greater degree compared with siRNA transfection alone (A and B). Altogether, down-regulation of FoxM1 enhanced PF-triggered anti-cancer activities in colon cancer cells.
Discussion
FoxM1 has been identified to play an oncogenic role in human colorectal cancer [Citation24–28]. For example, FoxM1 was reported to contribute to the development and growth of mouse colorectal cancer [Citation29]. Moreover, overexpression of FoxM1 was observed in tumor-cell nuclei of colorectal cancer and lymph node metastases [Citation30]. Overexpression of FoxM1 promoted the growth and metastasis in orthotopic mouse models, while FoxM1 down-regulation did the opposite [Citation30]. Consistently, overexpression of FoxM1 contributed to the progression of colorectal cancer [Citation31,Citation32]. Furthermore, FoxM1 expression correlated with tumor invasion and a poor prognosis in colorectal cancer [Citation33]. FoxM1 exerts its oncogenic function in part through activation of urokinase-type plasminogen activator receptor expression in colorectal cancer [Citation30]. Down-regulation of FoxM1 inhibited the proliferation and metastasis through reversal of EMT (epithelial to mesenchymal transtion) in colon cancer cells [Citation34]. FoxM1-induced PRX3 regulated stemness and survival of colon cancer cells through maintenance of mitochondrial function [Citation23]. One study showed that FoxM1 transactivated PTTG1 and HSPA5 and promoted colorectal cancer cell migration and invasion [Citation35,Citation36]. FoxM1D promoted EMT and metastasis through ROCKs activation in colorectal cancer [Citation37]. Recently, it has been reported that miR-320 suppressed colorectal cancer and enhanced the cells sensitivity to chemo-radiotherapy via targeting FoxM1 [Citation38,Citation39]. In addition, miR-149 suppressed colorectal cancer cell migration and invasion by targeting FoxM1 [Citation40]. Notably, miR-149 increased the sensitivity of colorectal cancer cells to 5-fluorouracil through targeting FoxM1 [Citation41]. Taken together, FoxM1 plays an important oncogenic role in the development and progression of colorectal cancer, indicating that targeting FoxM1 could be a novel strategy for the treatment of human colorectal cancer.
PF inhibited cell proliferative activity through induction of cell cycle arrest and the Fas/Fas ligand-mediated apoptosis pathway in human non-small cell lung cancer cells [Citation42]. Wu et al. found that PF suppressed NF-κB activation through modulation of IκB alpha and enhanced 5-fluorouracil-induced apoptosis in human gastric carcinoma cells [Citation43]. Moreover, PF inhibited cell growth and induced apoptosis in cervical cancer cells via down-regulation of Bcl-2 expression and up-regulation of Bax and caspase-3 expressions [Citation44]. One study also showed that PF induced human hepatoma cell apoptosis via inhibition of prostaglandin E receptor EP2 [Citation45]. Several studies further demonstrated that PF inhibited cell proliferation in breast cancer cells [Citation21], and ultiple myeloma cells [Citation19]. Wang et al. reported that PF inhibited cell growth and induced cell cycle arrest at G1 phase via DNA damage ad activation of p53 in colorectal carcinoma HT29 cells [Citation46]. In line with these reports, our study showed that PF inhibited cell proliferation and induced apoptosis and cell cycle arrest in colorectal cancer HCT116 cells. Emerging evidence has suggested that PF could inhibit cell invasion in human cancer cells. For example, PF was reported to inhibit cell invasion and metastasis via suppression of MMP-9 and ERK and upregulation of E-cadherin in human hepatocellular carcinoma cells [Citation47], and pancreatic cancer cells [Citation18]. Our study indicated that PF could be an effective anti-invasive agent for inhibition of colorectal cancer cell invasion.
PF modulated multidrug resistance via the inhibition of NF-kappaB activation in human gastric cancer cells [Citation48]. PF potentiates the inhibitory effects of erlotinib in pancreatic cancer cell lines through reducing ErbB3 phosphorylation [Citation17]. In addition, PF inhibited proliferation and invasion through suppression of Notch-1 signaling pathway [Citation21]. PF induced cell growth inhibition and apoptosis through the proteasome-dependent degradation of STAT3 in glioma cells [Citation49]. One study showed that PF inhibited proliferation and promoted apoptosis via governing miR-29b and MMP-2 (matrix metalloproteinase-2) in multiple myeloma cells [Citation19]. Interestingly, it has been reported that PF prevented hypoxia-induced epithelial-mesenchymal transition in human breast cancer cells [Citation50]. Notably, PF inhibited cell proliferation through up-regulation of miR-124 and suppression of PI3K/Akt and STAT3 signaling in human gastric carcinoma cells [Citation51]. In the current study, we identified that PF exerted its anti-tumor activity via inhibition of FoxM1 in human colorectal cancer cells. Several other compounds have been discovered to inhibit the expression of FoxM1. For instance, thiazolidinediones and mithramycin suppressed FoxM1 in human cancer cells [Citation52]. Diindolylmethane inhibited migration and invasion of colorectal cancer cells via down-regulation of uPA and MMP9 [Citation53]. Our current study identified that PF could be a potential agent to inhibit the FoxM1 in colorectal cancer patients.
Materials and Methods
Reagents
Anti-FoxM1 (SC-502) and anti-tubulin (SC-5274) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-p21 (#2947) and anti-p27 (#2552) antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). PF (purity is greater than 98%) was purchased from the HuanYu Biotechnology Development Company (Beijing, China). MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Transwell inserts and Matrigel were purchased from BD Biosciences. Annexin apoptosis assay kit was purchased from Beyotime Biotechnology (Shanghai, China). Lipofectamine 2000 reagent was obtained by Invitrogen (Waltham, MA USA).
Cell culture
The human colon cancer HCT116 cells were obtained from ATCC Company (Manassas, VA, USA) and cultured in RPMI 1640 medium (Gibro Invitrogen) supplemented with 10 % fetal bovine serum (FBS), 2 mM glutamine, 1 mM sodium pyruvate, 100 μg/ml streptomycin and 100 U/ml penicillin. These cells were maintained in a 5 % CO2 atmosphere at 37°C.
MTT assay
HCT116 cells were seeded in 96-well plates (5 × 103 cells/well) for overnight. PF was dissolved in DMSO (dimethyl sulfoxide). Then, cells were treated with different concentrations of PF or DMSO as control group for 24, 48, and 72 hours. MTT assay was performed to determine the cell viability in colon cancer cells after different times. Briefly, 10 μl MTT solution (0.5 mg/ml) was added in cell culture medium and incubated for 4 h in 5% CO2 atmosphere at 37°C. The supernatant in each well was removed and added 100 μl DMSO. The absorption was measured at 490 nm.
Cell apoptosis assay
Colon cancer cells (5 × 105 cells/well) were seeded in 6-well plates. After overnight incubation, cells were treated with PF for 48 hours. Then, cells were harvested and washed by PBS and subsequently suspended in 500 μl binding buffer including 5 μl annexin V-FITC and 5 μl Propidium iodide (PI) for 15 minutes at room temperature in the dark. Cell apoptosis was measured by a FACScalibur flow cytometer (BD, Franklin Lakes, NJ, USA).
Cell cycle analysis
Colon cells were treated with PF and cultured for 48 h in RPMI 1640 medium. Then, cells were harvested and washed with PBS, and added 7% cold alcohol for overnight at 4°C. Cells were washed and suspended with PBS, and incubated with 100 μg/ml RNase and 40 mg/ml PI for 30 minutes at 4°C. Cell cycle was determined by a FACScalibur flow cytometer (BD, USA).
Transwell migration and invasion assay
The cells with PF treatments were seeded into an upper chamber of inserts with serum-free medium in a 24-well plate at a density of 1.0 × 105 cells/well. The complete medium was added in bottom chamber of inserts. For cell invasion assay, the Transwell inserts were precoated with Matrigel. Cells were incubated for 20 h in 5% CO2 atmosphere at 37°C. Then, the upper cells in chamber were removed by the cotton buds and the bottom surface cells were stained with 4μg/ml Calcein AM at 37ºC for 1 h. These fluorescently labeled invasive cells were photographed by a fluorescent microscope.
Wound healing assay
After HCT116 cells grew to 90% confluence, a rectangular lesion on monolayers was created with a sterile 100 μl pipette tip. Cells were treated with PF and cultured for 20 hours. Photographic images were taken at the lesion border using an inverted microscope (Olympus, IX71).
Quantitative real-time reverse transcription-PCR (Q-PCR) analysis
The total RNA was extracted with Trizol reagent and reversed-transcribed into cDNA by First Strand cDNA Synthesis Kit. Real-time Q-PCR was performed using Power SYBR Green PCR Master Mix and the results were calculated as described before [Citation54]. The primers for FoxM1 were as follows: forward primer: 5’– AAC CGC TAC TTG ACA TTG G -3’; and reverse primer 5’- GCA GTG GCT TCA TCT TCC -3’. And primers for GAPDH: forward primer: 5′- ACC CAG AAG ACT GTG GAT GG -3′; reverse primer: 5′- CAG TGA GCT TCC CGT TCA G- 3′.
Western blotting analysis
The cells were harvested and lysed in RIPA buffer with protease inhibitors and phosphatase inhibitors (EMD Millipore, Billerica, MA, USA). The equal quantity of proteins were loaded on SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and subsequently transferred to PVDF (polyvinylidene fluoride) membranes for Western blotting as described earlier [Citation55]. ImageJ software was used for densitometric quantification of the protein blots.
Transfection
HCT116 cells (3 × 105 cells/well) were cultured in 6-well plates, and transfected with FoxM1 cDNA (OriGene technologies; Rockville, MD) or FoxM1 siRNA (GenePharma Company, Shanghai) or empty vector using lipofectamine 2000 following the manufacturer's instructions [Citation56].
Statistical analysis
The data were presented as the mean values ± SE. Comparisons between groups were evaluated by Student's t test. p < 0.05 was considered to be statistically significant.
Disclosure of Potential Conflicts of Interest
There is no conflict of interest.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. PMID:28055103
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. PMID:28248415
- Stintzing S, Tejpar S, Gibbs P, et al. Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur J Cancer. 2017;84:69–80. doi:10.1016/j.ejca.2017.07.016. PMID:28787661
- Gartel AL. FOXM1 in cancer: interactions and vulnerabilities. Cancer Res. 2017;77:3135–3139. doi:10.1158/0008-5472.CAN-16-3566. PMID:28584182
- Bella L, Zona S, Nestal de Moraes G, et al. FOXM1: A key oncofoetal transcription factor in health and disease. Semin Cancer Biol. 2014;29:32–39. doi:10.1016/j.semcancer.2014.07.008. PMID:25068996
- Laoukili J, Stahl M, Medema RH. FoxM1: at the crossroads of ageing and cancer. Biochim Biophys Acta. 2007;1775:92–102. PMID:17014965
- Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem. 2007;388:1257–1274. doi:10.1515/BC.2007.159. PMID:18020943
- Huang C, Du J, Xie K. FOXM1 and its oncogenic signaling in pancreatic cancer pathogenesis. Biochim Biophys Acta. 2014;1845:104–116. PMID:24418574
- Li L, Wu D, Yu Q, et al. Prognostic value of FOXM1 in solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8:32298–32308. PMID:28427178
- Quan M, Wang P, Cui J, et al. The roles of FOXM1 in pancreatic stem cells and carcinogenesis. Mol Cancer. 2013;12:159. doi:10.1186/1476-4598-12-159. PMID:24325450
- Wierstra I. FOXM1 (Forkhead box M1) in tumorigenesis: overexpression in human cancer, implication in tumorigenesis, oncogenic functions, tumor-suppressive properties, and target of anticancer therapy. Adv Cancer Res. 2013;119:191–419. doi:10.1016/B978-0-12-407190-2.00016-2. PMID:23870513
- Shi M, Cui J, Xie K. Signaling of miRNAs-FOXM1 in cancer and potential targeted therapy. Curr Drug Targets. 2013;14:1192–1202. doi:10.2174/13894501113149990192. PMID:23834153
- Wierstra I. The transcription factor FOXM1 (Forkhead box M1): proliferation-specific expression, transcription factor function, target genes, mouse models, and normal biological roles. Adv Cancer Res. 2013;118:97–398. doi:10.1016/B978-0-12-407173-5.00004-2. PMID:23768511
- Halasi M, Gartel AL. Targeting FOXM1 in cancer. Biochem Pharmacol. 2013;85:644–652. doi:10.1016/j.bcp.2012.10.013. PMID:23103567
- Chen A, Wang H, Zhang Y, et al. Paeoniflorin exerts neuroprotective effects against glutamateinduced PC12 cellular cytotoxicity by inhibiting apoptosis. Int J Mol Med. 2017;40:825–833. PMID:28731183
- Gu P, Zhu L, Liu Y, et al. Protective effects of paeoniflorin on TNBS-induced ulcerative colitis through inhibiting NF-kappaB pathway and apoptosis in mice. Int Immunopharmacol. 2017;50:152–160. doi:10.1016/j.intimp.2017.06.022. PMID:28666238
- Hao J, Yang X, Ding XL, et al. Paeoniflorin potentiates the inhibitory effects of erlotinib in pancreatic cancer cell lines by reducing ErbB3 phosphorylation. Sci Rep. 2016;6:32809. doi:10.1038/srep32809. PMID:27609096
- Yang N, Cui H, Han F, et al. Paeoniflorin inhibits human pancreatic cancer cell apoptosis via suppression of MMP-9 and ERK signaling. Oncol Lett. 2016;12:1471–1476. PMID:27446455
- Wang S, Liu W. Paeoniflorin inhibits proliferation and promotes apoptosis of multiple myeloma cells via its effects on microRNA29b and matrix metalloproteinase2. Mol Med Rep. 2016;14:2143–2149. doi:10.3892/mmr.2016.5498. PMID:27430753
- Li Y, Gong L, Qi R, et al. Paeoniflorin suppresses pancreatic cancer cell growth by upregulating HTRA3 expression. Drug Des Devel Ther. 2017;11:2481–2491. doi:10.2147/DDDT.S134518. PMID:28860718
- Zhang Q, Yuan Y, Cui J, et al. Paeoniflorin inhibits proliferation and invasion of breast cancer cells through suppressing Notch-1 signaling pathway. Biomed Pharmacother. 2016;78:197–203. doi:10.1016/j.biopha.2016.01.019. PMID:26898442
- Zhong S, Zhou A, Qi F, et al. Downregulating forkhead box M1 inhibits proliferation by inhibiting autophagy in the sw480 cell line. Biomed Rep. 2017;7:47–50. doi:10.3892/br.2017.915. PMID:28685059
- Song IS, Jeong YJ, Jeong SH, et al. FOXM1-Induced PRX3 Regulates Stemness and Survival of Colon Cancer Cells via Maintenance of Mitochondrial Function. Gastroenterology. 2015;149:1006–1016 e9. doi:10.1053/j.gastro.2015.06.007. PMID:26091938
- Zhang J, Zhang K, Zhou L, et al. Expression and potential correlation among Forkhead box protein M1, Caveolin-1 and E-cadherin in colorectal cancer. Oncol Lett. 2016;12:2381–2388. PMID:27698803
- Zhang C, Wang Y, Feng Y, et al. Gli1 promotes colorectal cancer metastasis in a Foxm1-dependent manner by activating EMT and PI3K-AKT signaling. Oncotarget. 2016;7:86134–86147. PMID:27863385
- Valverde A, Penarando J, Canas A, et al. The addition of celecoxib improves the antitumor effect of cetuximab in colorectal cancer: role of EGFR-RAS-FOXM1-beta- catenin signaling axis. Oncotarget. 2017;8:21754–21769. PMID:28423516
- Wang D, Hu G, Du Y, et al. Aberrant activation of hedgehog signaling promotes cell proliferation via the transcriptional activation of forkhead Box M1 in colorectal cancer cells. J Exp Clin Cancer Res. 2017;36:23. doi:10.1186/s13046-017-0491-7. PMID:28148279
- Xie T, Geng J, Wang Y, et al. FOXM1 evokes 5-fluorouracil resistance in colorectal cancer depending on ABCC10. Oncotarget. 2017;8:8574–8589. PMID:28051999
- Yoshida Y, Wang IC, Yoder HM, et al. The forkhead box M1 transcription factor contributes to the development and growth of mouse colorectal cancer. Gastroenterology. 2007;132:1420–1431. doi:10.1053/j.gastro.2007.01.036. PMID:17408638
- Li D, Wei P, Peng Z, et al. The critical role of dysregulated FOXM1-PLAUR signaling in human colon cancer progression and metastasis. Clin Cancer Res. 2013;19:62–72. doi:10.1158/1078-0432.CCR-12-1588. PMID:23136192
- Zhang H, Zhong H, Li L, et al. Overexpressed transcription factor FOXM1 contributes to the progression of colorectal cancer. Mol Med Rep. 2016;13:2696–2700. doi:10.3892/mmr.2016.4875. PMID:26861549
- Zhang HG, Xu XW, Shi XP, et al. Overexpression of forkhead box protein M1 (FOXM1) plays a critical role in colorectal cancer. Clin Transl Oncol. 2016;18:527–532. doi:10.1007/s12094-015-1400-1. PMID:26370421
- Chu XY, Zhu ZM, Chen LB, et al. FOXM1 expression correlates with tumor invasion and a poor prognosis of colorectal cancer. Acta Histochem. 2012;114:755–762. doi:10.1016/j.acthis.2012.01.002. PMID:22326401
- Yang K, Jiang L, Hu Y, et al. Short hairpin RNA- mediated gene knockdown of FOXM1 inhibits the proliferation and metastasis of human colon cancer cells through reversal of epithelial-to-mesenchymal transformation. J Exp Clin Cancer Res. 2015;34:40. doi:10.1186/s13046-015-0158-1. PMID:25935853
- Zheng Y, Guo J, Zhou J, et al. FoxM1 transactivates PTTG1 and promotes colorectal cancer cell migration and invasion. BMC Med Genomics. 2015;8:49. doi:10.1186/s12920-015-0126-9. PMID:26264222
- Luo X, Yao J, Nie P, et al. FOXM1 promotes invasion and migration of colorectal cancer cells partially dependent on HSPA5 transactivation. Oncotarget. 2016;7:26480–26495. doi:10.18632/oncotarget.8419. PMID:27034162
- Zhang X, Zhang L, Du Y, et al. A novel FOXM1 isoform, FOXM1D, promotes epithelial-mesenchymal transition and metastasis through ROCKs activation in colorectal cancer. Oncogene. 2017;36:807–819. doi:10.1038/onc.2016.249. PMID:27399334
- Wan LY, Deng J, Xiang XJ, et al. miR-320 enhances the sensitivity of human colon cancer cells to chemoradiotherapy in vitro by targeting FOXM1. Biochem Biophys Res Commun. 2015;457:125–132. doi:10.1016/j.bbrc.2014.11.039. PMID:25446103
- Vishnubalaji R, Hamam R, Yue S, et al. MicroRNA-320 suppresses colorectal cancer by targeting SOX4, FOXM1, and FOXQ1. Oncotarget. 2016;7:35789–35802. doi:10.18632/oncotarget.8937. PMID:27119506
- Xu K, Liu X, Mao X, et al. MicroRNA-149 suppresses colorectal cancer cell migration and invasion by directly targeting forkhead box transcription factor FOXM1. Cell Physiol Biochem. 2015;35:499–515. doi:10.1159/000369715. PMID:25613903
- Liu X, Xie T, Mao X, et al. MicroRNA-149 Increases the Sensitivity of Colorectal Cancer Cells to 5-Fluorouracil by Targeting Forkhead Box Transcription Factor FOXM1. Cell Physiol Biochem. 2016;39:617–629. doi:10.1159/000445653. PMID:27415661
- Hung JY, Yang CJ, Tsai YM, et al. Antiproliferative activity of paeoniflorin is through cell cycle arrest and the Fas/Fas ligand-mediated apoptotic pathway in human non-small cell lung cancer A549 cells. Clin Exp Pharmacol Physiol. 2008;35:141–147. doi:10.1111/j.1440-1681.2008.04935.x. PMID:17941899
- Wu H, Li W, Wang T, et al. Paeoniflorin suppress NF-kappaB activation through modulation of I kappaB alpha and enhances 5-fluorouracil-induced apoptosis in human gastric carcinoma cells. Biomed Pharmacother. 2008;62:659–666. doi:10.1016/j.biopha.2008.08.002. PMID:18809274
- Zhang L, Zhang S. Modulating Bcl-2 family proteins and caspase-3 in induction of apoptosis by paeoniflorin in human cervical cancer cells. Phytother Res. 2011;25:1551–1557. doi:10.1002/ptr.3534. PMID:21698669
- Hu S, Sun W, Wei W, et al. Involvement of the prostaglandin E receptor EP2 in paeoniflorin-induced human hepatoma cell apoptosis. Anticancer Drugs. 2013;24:140–149. doi:10.1097/CAD.0b013e32835a4dac. PMID:23069790
- Wang H, Zhou H, Wang CX, et al. Paeoniflorin inhibits growth of human colorectal carcinoma HT 29 cells in vitro and in vivo. Food Chem Toxicol. 2012;50:1560–1567. doi:10.1016/j.fct.2012.01.035. PMID:22326807
- Lu JT, He W, Song SS, et al. Paeoniflorin inhibited the tumor invasion and metastasis in human hepatocellular carcinoma cells. Bratisl Lek Listy. 2014;115:427–433. PMID:25077366
- Fang S, Zhu W, Zhang Y, et al. Paeoniflorin modulates multidrug resistance of a human gastric cancer cell line via the inhibition of NF-kappaB activation. Mol Med Rep. 2012;5:351–356. PMID:22051979
- Nie XH, Ou-yang J, Xing Y, et al. Paeoniflorin inhibits human glioma cells via STAT3 degradation by the ubiquitin-proteasome pathway. Drug Des Devel Ther. 2015;9:5611–5622. PMID:26508835
- Zhou Z, Wang S, Song C, et al. Paeoniflorin prevents hypoxia-induced epithelial-mesenchymal transition in human breast cancer cells. OncoTargets and therapy. 2016;9:2511–2518. doi:10.2147/OTT.S102422. PMID:27175085
- Zheng YB, Xiao GC, Tong SL, et al. Paeoniflorin inhibits human gastric carcinoma cell proliferation through up-regulation of microRNA-124 and suppression of PI3K/Akt and STAT3 signaling. World J Gastroenterol. 2015;21:7197–7207. doi:10.3748/wjg.v21.i23.7197. PMID:26109806
- Petrovic V, Costa RH, Lau LF, et al. Negative regulation of the oncogenic transcription factor FoxM1 by thiazolidinediones and mithramycin. Cancer biology & therapy. 2010;9:1008–1016. doi:10.4161/cbt.9.12.11710.
- Jin H, Li XJ, Park MH, et al. FOXM1-mediated downregulation of uPA and MMP9 by 3,3'-diindolylmethane inhibits migration and invasion of human colorectal cancer cells. Oncol Rep. 2015;33:3171–3177. doi:10.3892/or.2015.3938. PMID:25962429
- Yin X, Zhang Y, Su J, et al. Rottlerin exerts its anti-tumor activity through inhibition of Skp2 in breast cancer cells. Oncotarget. 2016;7:66512–66524. doi:10.18632/oncotarget.11614. PMID:27582552
- Huang Y, Zhao M, Xu H, et al. RASAL2 down-regulation in ovarian cancer promotes epithelial-mesenchymal transition and metastasis. Oncotarget. 2014;5:6734–6745. doi:10.18632/oncotarget.2244. PMID:25216515
- Wang L, Hou Y, Yin X, et al. Rottlerin inhibits cell growth and invasion via down-regulation of Cdc20 in glioma cells. Oncotarget. 2016;7:69770–69782. PMID:27626499