ABSTRACT
S100A4 is a Ca2+-binding protein that performs an important role in metastasis. It is also known for its antitumor functions. S100A4 is expressed by a specialized subset of CD4+CD25+ lymphocytes and is present on those cell's membranes along with peptidoglycan recognition proteins (PGRPs). There, by interacting with major heat shock protein Hsp70, S100A4 plays an important cytotoxic role. The resulting stably formed complex of PGRPs, S100A4 and Hsp70 is required for the identification and binding between a lymphocyte and a target cell. Here, we investigated the S100A4 functions in CD4+CD25+PGRPs+S100A4+ lymphocyte cytotoxicity against target cells. We demonstrated that those lymphocytes do not form a stable complex with the tumor target cells that themselves have S1004A on their surface. That observation can be explained by our finding that S100A4 precludes the formation of a stable complex between PGRPs, S100A4 (on the lymphocytes’ surface), and Hsp70 (on the target cells’ surface). The decrease in S100A4 level in CD4+CD25+PGRPs+S100A4+ lymphocytes inhibits their cytotoxic activity, while the addition of S100A4 in the medium restores it. Thus, the resistance of target cells to CD4+CD25+PGRPs+ S100A4+ lymphocyte cytotoxicity depends on their S100A4 expression level and can be countered by S100A4 antibodies.
1. Introduction
S100A4 protein belongs to a Ca2+-binding protein family and was first described as MTS1 protein that plays an important role in controlling cancer metastasis in mice. Particularly, the experiments with cancer cells expressing MTS1 demonstrated a direct correlation between MTS1 gene expression and metastatic phenotype [Citation1]. Further studies verified the role of S100A4 protein in cancer biology, for instance, the increased S100A4 expression among those with breast cancer is highly correlated with survival probability of those patients [Citation2].
S100A4 was shown to be most extensively present in human bone marrow, spleen, peritoneal macrophages, and lymphocytes. S100A4 is involved in the cell motility regulation of various cell types, particularly macrophages and T-lymphocytes [Citation1]. The major part of intracellular S1000A4 is diffusely distributed in a cytoplasm [Citation3].
Additionally, S100A4 is present on a cellular membrane [Citation4] and is also secreted into an extracellular medium. It has been established that S100A4 is secreted both by cancer and stromal cells. S1000A4 triggers pro-metastatic cascades by modifying cancer cells’ cytoskeletons and focal adhesions [Citation5]. S100A4 is also secreted by neutrophils and lymphocytes as the complex with tag7/PGRPs serving in that capacity as a chemoattractant for natural killer cells [Citation6].
Recently beta-catenin has been demonstrated as a direct activator of S100A4 transcription [Citation7]. Transcriptional factor Oct-1 is also known to be a S100A4 expression regulator responsible for the redistribution of intra- versus extracellular S100A4 protein pools [Citation8]. At the same time, anticancer compound paclitaxel (active pharmaceutical ingredient of Taxol) is demonstrated to significantly inhibit S100A4 expression [Citation9]. Before that, the drug was considered as a solely cytotoxic remedy with antimitotic activity. More recent findings establish that intra-cancer paclitaxel concentrations are too low to cause a mitotic arrest [Citation10]. Thus, S100A4 level regulation in cancer cells is a potentially new direction for cancer drug treatment.
Besides its role in metastatic processes, S100A4 is also involved in defense against cancer cells. Thus, S100A4 demonstrates a multidirectional impact on host-tumor interactions. For instance, the presence of S100A4 was demonstrated in the T-lymphocytes clusters that surround carcinomas and their metastases [Citation11]. S100A4 was found in the specialized subset of CD4+CD25+ lymphocytes where the protein is present on the cellular membranes as a complex with Tag7/PGRPs [Citation12]. Currently established is the mechanism for S100A4 participation in CD4+CD25+ lymphocyte cytotoxicity against cancer cells that do not express S100A4 [Citation13]. Specifically, lymphocyte-derived S100A4 together with Tag7/PGRPs forms a stable complex with Hsp70 on the surface of target cells during a lymphocyte-target cell contact. This complex formation triggers FasL/Fas-induction with consequent apoptosis of cancer cells [Citation12]. The studies of paclitaxel‘s impact on the cytotoxicity of interleukin-2 activated lymphocytes demonstrated the decrease in their cytotoxic activity against К562 cell line (at the same time paclitaxel was not directly toxic to the lymphocytes) [Citation14]. It might be hypothesized that the decreased lymphocyte cytotoxicity is associated with the decrease of S100A4 expression in those cells due to the effects of paclitaxel.
The aim of this work is to identify the role of S100A4 in cytotoxic activity of CD4+CD25+ PGRPs+S100A4+ lymphocytes against cancer cells that (1) do not express S100A4 (S100A4−) and (2) do express S100A4.
2. Materials and methods
Cells, proteins and antibodies. Human erythroblastoid K562, mouse adenocarcinoma CSML-100, and human melanoma M3 cells were cultured in RPMI 1640 supplemented with 2 mM L-glutamine and 10% fetal calf serum (all Gibco BRL).
Human peripheral blood was obtained from healthy volunteers. Lymphocytes were isolated by Ficoll–Hypaque gradient centrifugation and cultivated for 6 d with 1000 U/ml of recombinant interleukin-2 (Sigma) or paclitaxel 10 μg/ml (Sigma). Lymphocyte subsets were obtained using commercially available magnetic bead isolation kits (Dynal Biotech ASA) following the manufacturer's protocols.
The cDNAs for recombinant mouse Tag7, mouse Mts1/S100A4, and human Hsp70 were subcloned in pQE-31 and expressed in Escherichia coli M15[pREP4] (Qiagen). The proteins were purified on Ni-NTA agarose (Qiagen) as recommended by the manufacturer. Rabbit antibodies raised against recombinant Tag7, Mts1 or Hsp70 were affinity-purified on Sepharose 4B (Amersham-Pharmacia Biotech) CNBr-coupled with rTag7, rMts1 and rHsp70 following the manufacturer's manual. Rabbit polyclonal antibodies affinity-purified on the corresponding antigens were coupled to cyanogen bromide-activated Sepharose following the standard protocol. S100A4 content in the samples was measured by competitive EIA [Citation15].
2.1. Biotinylation and chemical cross-linking
Escherichia coli-expressed recombinant human Hsp70 and mouse Mts1/S100A4 (40 mkg/ml) were incubated for 2 hours at room temperature with Sulfo-NHS-biotin (Pierce) at a 1:100 molar ratio. The reaction was stopped by the addition of Tris HCl (pH 8.0) and the sample was extensively dialyzed at 4oC against PBS. Lymphocytes at 5 × 107 were resuspended in PBS containing 50 mM hepes (pH 8.3) and 0.2 mM BS3 (Pierce) and incubated with biotinylated Hsp70, Mts1 for 30 min at 4oC. The cells then were washed twice PBS.
For biotinylation, CSML100 cells were washed three times with ice-cold PBS to remove contaminating fetal calf serum and other proteins from the culture medium, before suspending at 25 × 106 cells/ml in PBS (pH 8.0) and reacting with 0.5 mg/ml sulfo-NHS-biotin for 30 min at room temperature. The cells were then washed twice with cold PBS to remove unreacted biotin before solubilization.
2.2 Purification of membrane proteins CSML-100 cells
For obtained membrane-bound proteins, the cells were solubilized at 2.5 × 107 cells/ml in lysis buffer containing 1% triton X-100, 20 mM Tris HCl, pH 7.6, 150 mM NaCl, and protease inhibitor (10 µg/ml leupeptin, 10 µg/ml antipain, and 10 µg/ml pepstatin, all from Sigma). Solubilizations were carried out for 30 min on ice with occasional vortexing. The lysates were centrifuged for 15 min at 10,000g in an eppendorf centrifuge at 4oC and 45 min at 100,000g in an ultracentrifuge at 4o C, then membrane proteins were solubilized from pellets with either. The soluble membrane proteins were purified with 1M KCl as described.
2.3 Immunoprecipitations and immunoblotting
The membrane proteins were purified by anti-Tag7-seprarose chromatography [Citation4]. Protein concentration was determined by the Bradford assay. Proteins were fractioned by SDS-PAGE, transferred to nitrocellulose and analyzed with ECL Streptavidin-Horseradish Peroxidase conjugate (Amersham Biosciences).
2.4. Cytotoxicity
Cells were cultured in 96-well plates at a density of 3 × 104 cells/well, then lymphocytes (20:1) were added in 100 µl and incubated for 3 hours at 37°C. In inhibition tests, antibodies were used at 10 and 20 µg/ml. Cell death was determined by an MTT test.
2.5 Microscopy
To visualize cell contacts, K562 cells (Fas) and CD4+CD25+ (1:20) were incubated in RPMI 1640 for 30 minutes, washed twice with PBS, and fixed with 4% formaldehyde (Sigma) for 20 minutes at 4°C. Then, cells were washed and stained in PBS with rabbit anti-S100A4 antibodies (Neo Markers) followed by FITC-labeled goat anti-rabbit IgG (Sigma), and with phycoerythrin-labeled anti-CD95 (anti-Fas) antibody (Caltag Laboratories). After washing with 50 mM NH4Cl, stained material was bound to polylysine-treated coverslips. Fluorescence images were obtained with a Leica TCS SP2 confocal microscope, analyzed with Leica confocal software, and prepared in Photoshop CE (Adobe Systems, San Jose, CA). Confocal images were quantified by ImageJ software analysis.
2.6 Statistical analysis
A Student t test for means (paired 2 samples) was used to calculate significance; p values <0.05 were considered significant.
3. Results
3.1 S100A4 on CD4+CD25+PGRPs+S100A4+ lymphocytes’ membranes (S100A4L) partakes in their contact with S100A4− target cells by remaining on those cells’ surface after the contact.
It has been demonstrated earlier, that PGRPs from the surface of CD4+CD25+PGRPs+S100A4+ lymphocytes (PGRPsL) remain on the surface of the target cell after the lymphocyte-target cell contacts [Citation4]. Here, we showed the similar outcome with regards to S100A4L protein in the same contact situation.
Human erythroblastoid K562 were incubated with CD4+CD25+PGRPs+S100A4+ lymphocytes for the duration of 18 hours. The lethality among target cells during this incubation was consistent with other studies and was 20% with respect to the control cells [Citation4].
Confocal microscopy imaging () demonstrates that S100A4L (stained in green) is evenly distributed on the lymphocyte surface before contacts with a target cell (A). B shows a К562 cell (S100A4−) stained in red. On C the same K562 cell is surrounded by lymphocytes after they have contacted. It can be observed that S100A4L (green) from the lymphocytes’ surface relocated onto the K562 surface. At the same time, S100A4L concentration in the lymphocytes that surround the cell target was significantly decreased: mean lymphocytes’ fluorescence was decreased 4 times, p<0.001 (see C versus 1A).
Figure 1. Relocation of S100A4 protein from lymphocytes to the surface of cancer target cell. A,B. Double immunostaining for S100A4 and CD95 revealed exclusive expression of S100A4 protein in lymphocytes (green), while CD95 expression was limited to K562 cell before cell contact (red). С. Immunostaining with anti-S100A4 antibodies detected lymphocytes as well as cancer cell. (10.0x/1.40 NA oil objective). D. Co-localization of S100A4 and CD95 on the surface of K562 target cell after contact with lymphocytes.
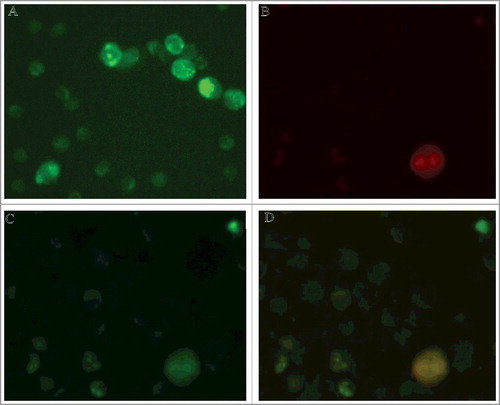
Those observations establish that S100A4L, similarly to PGRPsL[13], remained on the surface of the target cell after the intercellular contact was dissolved. It indicates that S100A4L is tightly associated with the complex of PGRPsL and Hsp70 on the target cell surface (Hsp70C).
3.2 No CD4+CD25+PGRPs+S100A4+ lymphocyte cytotoxicity towards target cells containing S100A4 on their membranes (S100A4C)
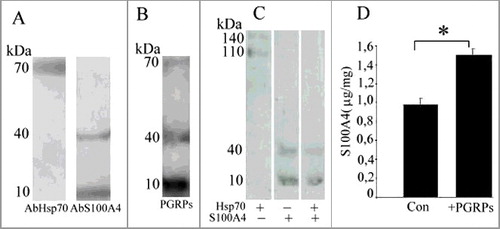
CD4+CD25+PGRPs+S100A4+ lymphocyte cytotoxicity was described against target cells that do not express S100A4. Since cancer cells in some cases do contain S100A4С, we were interested to investigate CD4+CD25+PGRPs+S100A4+ lymphocyte cytotoxicity against this type of cells. In our studies we used CSML-100 cell line that has a high S100A4C expression level [Citation16]. On the cell's surface, S100A4C was present as both monomer (10 kDa) and tetramer (40 kDA) (A) consistent with the S100A4L migration pattern demonstrated for the lymphocyte protein [Citation4].
Figure 2. А. Western-blot analysis of CSML-100 surface proteins. В. Western-blot analysis of CSML-100 surface proteins after their interaction with PGRPs. C. Western-blot analysis of recombinant proteins: Hsp70, S100A4, Hsp70 and S100A4 after their interaction with a lymphocyte. А and В. CSML-100 surface proteins were biotinylated; results are visualized using Streptavidin-Horseradish Peroxidase. C. Recombinant Hsp70 and S100A4 are biotinylated; formed complexes are extracted on AbPGRPs-sepharose. D. S100A4 concentration on the CSML-100 cells before and after their stimulation with PGRPs. The S100A4 content in the samples was measured by ELISA. The stimulation was performed by adding 20 ng/ml PGRPs into the CSML-100 incubation medium for 24 hours. Error bars show SEM based on five biological replicates.
We established that CD4+CD25+PGRPs+S100A4+ lymphocyte cytotoxicity against CSML-100 cells was no more than 2–3%.
To determine themechanism behind that finding, we investigated whether a stable complex of PGRPsL, S100A4L, S100A4C, and Hsp70C can be formed under such conditions. This complex is required for both the contact between a cytotoxic lymphocyte and target cell and for the subsequent cancer cell lysis.
First, we examined if Hsp70С proteins and S100A4С on the surface of CSML-100 can interact with PGRPsL. The surface CSML-100 proteins were biotinylated and extracted from the cells’ surface using 1M KCl. Further, the extracted proteins were transferred onto recPGRPs sepharose; the bound proteins were detected during a western-blot analysis as molecular weight bands of 70, 40, and 10 kDa (B). Among those, the 70-kDa band represented Hsp70 protein, while 10-kDa- and 40-kDa bands represented S100A4 monomer and tetramer respectively. Thus, Hsp70C and S100A4C proteins from the surface of CSML-100 cells are able to interact with PGRPsL.
3.3 S100A4 replaces Hsp70 in their complex with PGRPs
Second, we investigated potential molecular mechanisms for S100A4С on the surface of cancer cells to preclude the formation of PGRPsL, S100A4L, and Hsp70C intercellular complex. To do so, we analyzed molecular complexes that recHsp70 and recS100A4 form if they added to the incubation medium with PGRPsL and S100A4L proteins from the surface of CD4+CD25+ PGRPs+S100A4+ lymphocytes. We added biotinylated recombinant proteins recHsp70, or recS100A4, or their combination to the lymphocytes. After co-incubation in the presence of BS3 crosslinking reagent, we extracted the surface proteins of CD4+CD25+PGRPs+S100A4+ lymphocytes as well as the biotinylated proteins bound to those surface proteins on antiPGRPs-sepharose.
C illustrates that, if only recHsp70 is added, PGRPsL and S100A4L lymphocyte proteins form stable triple complexes visible as molecular weight bands of 110 kDa and 140 kDa. If only recS100A4 is added, PGRPsL binds both recS100A4 biotinylated forms (of the molecular weights 10 and 40 kDa). However, when the both biotinylated recHsp70 and recS100A4 are added, PGRPsL binds only with recS100A4 (the 10-kDa and 40-kDa bands are visible on the gel). That finding indicates that the presence of recS100A4 precludes the interaction between PGRPsL and recHsp70 and inhibits the formation of the functional protein complex.
S100A4C expression can be regulated. For instance, CSML-100 cells react to the addition of recPGRPs into medium by the increased expression of S100A4. D illustrates that the incubation of CSML-100 cells in the presence of recPGRPs stimulated the 1.5-fold S100A4C increase. Such increased S100A4C expression can be one of the cancer cells’ defense mechanisms.
3.4 Target cells’ resistance depends on S100A4C expression level; the addition of S100A4 antibodies decreases that resistance
For further investigation of the relation between S100A4C expression in cancer cells and their resistance to CD4+CD25+PGRPs+S100A4+ lymphocytes’ cytotoxicityin addition to CSML-100 cell line we utilized М3 melanoma cell line that also expresses S100A4C.
The incubation of CD4+CD25+PGRPs+S100A4+ lymphocytes with CSML-100 or melanoma М3 cells lead to 3% lethality among CSML-100 and 30% lethality among melanoma М3 cells (A).
Figure 3. A. S100A4 impact on target cells survival. The activated lymphocytes (20:1) were added to CSML-100 and melanoma M3 cells that were pre-cultivated with S100A4 antibodies and incubated for 3 hours at 37°C. The cells survival was measured with an MTT test in five independent experiments. Error bars are ±SEM. B. The lymphocytes were extracted from the peripheral blood of healthy donors and incubated for 6 d with 1000 U/ml interleukin-2 or with interleukin-2 and 10 μ/ml paclitaxel. Cultivated and not cultivated with paclitaxel lymphocytes were added to К562 cells in the presence of 1 mM recS100A4, and in the presence or absence of 1mM Ca2+. *p < 0.05. Error bars show SEM based on five biological replicates. С. Paclitaxel impact on CSML-100 cells survival in the presence of cultivated and not cultivated with paclitaxel lymphocytes. The part of CSML-100 cells was pre-cultivated with paclitaxel (10 μ/ml), and then cultivated and not cultivated with paclitaxel lymphocytes were added. Error bars show SEM based on five biological replicates, *p < 0.05.
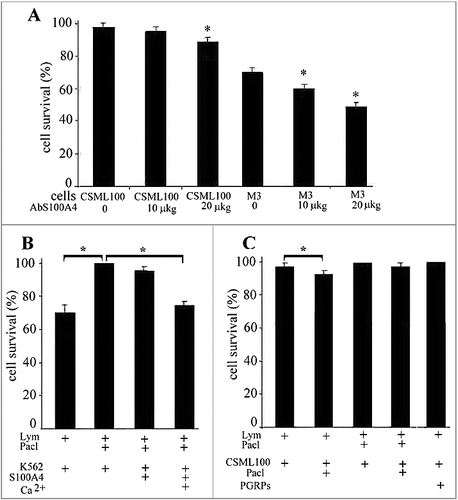
Lastly, to block S100A4C, we added neutralizing antibodies in two increasing concentrations (10 and 20 µg/ml) into the condition medium. Incubation with the S100A4 antibodies led to an increasing dose-dependent lymphocyte cytotoxicity against CSML100 and М3 melanoma cells. Specifically, under the antibodies concentration of 20µg/ml, the cytotoxicity increased to 10% for CSML-100 and to 51% for melanoma М3 cells (А). Thus, the cancer cells’ resistance to the cytotoxic lymphocytes directly depends on the presence of S100A4С on the cancer cell's surface, and the S100A4C blockage by the antibodies increases the lethality among cancer cells.
3.5 The decrease of S100A4L level in CD4+CD25+PGRPs+S100A4+ lymphocytes inhibits their cytotoxic activity
Finally, we investigated if the level of S100A4L plays the role in the cytotoxic activity of CD4+CD25+PGRPs+S100A4+ lymphocytes. We tested paclitaxel's impact on lymphocytes cytotoxicity and investigated if the cytotoxicity inhibition caused by paclitaxel can be overcome by adding recS100A4 into the medium.
To accomplish that, К562 cells were incubated with CD4+CD25+PGRPs+S100A4+ lymphocytes pre-cultivated with paclitaxel (10 μg/ml). That incubation ultimately decreased the lymphocyte's cytotoxicity from 30% to 0% (B). Under a parallel experiment, during that incubation with the target cells, recS100A4 was added into the medium. The addition of 1 mM of recS100A4 into the medium led to the partial preservation of the cytotoxicity at the 5% level.
Under the inhibition of endogenous S100A4L expression, recS100A4 can potentially interact with both PGRPsL, partially replacing the endogenous S100A4L, as well as with Hsp70C on the target cells’ surface. The addition of 1mM of Са2+ during that incubation preserved the cytotoxicity at the level of 25% (B). Са2+ was previously demonstrated to lead to an increase of the binding constant between S100A4 and PGRPs (the apparent dissociation constants in the absence and presence of Ca2+ were 2 × 10—8 M and 10—9 M, respectively) [Citation12].
Thus, the paclitaxel-caused inhibition of CD4+CD25+PGRPs+S100A4+ lymphocyte cytotoxicity can be a result of the S100A4L level decrease and subsequent malfunctioning of the S100A4L interaction with PGRPsL on a lymphocyte membrane. This paclitaxel's inhibitory effect is reversed by the recS100A4 addition to the medium (in the presence of Са2+).
The use of paclitaxel during the CSML-100 cell incubation led to a decrease in S100A4C expression in those cells, and, as a result, the lymphocytes pre-cultivated with paclitaxel regained their cytotoxicity (from 0% to 3%) against CSML-100 cells (С). Thus, the decrease in S100A4C level in cancer cells leads to their higher susceptibility to CD4+CD25+PGRPs+S100A4+ lymphocyte cytotoxicity. The use of paclitaxel for both the lymphocytes and the target cells restores the cytotoxicity (C).
Since earlier we observed the increase in S100A4 in CSML-100 cells after their stimulation with PGRPs (D), we measured the lymphocyte cytotoxicity against the CSML-100 cells pre-cultivated with recPGRPs. This cytotoxicity ultimately decreased to the 0% level as expected (C).
4. Discussion
Our study investigates the role of S100A4 in the interaction between the cytotoxic lymphocytes and target cells. Here, we analyzed the impact of CD4+CD25+PGRPs+S100A4+ lymphocytes on target cells that express S100A4+ of the varying level. We demonstrated that CD4+CD25+PGRPs+S100A4+ lymphocytes are not cytotoxic against the cells that express S100A4+. We also analyzed reasons behind that absence of cytotoxicity. Specifically, we established that S100A4 on the lymphocyte surface, similarly to PGRPs, remains on the surface of the target cell after the contact. Thus, S100A4 expression provides an opportunity for cancer cells to avoid CD4+CD25+PGRPs+S100A4+ lymphocyte cytotoxicity. On the other hand, the absence of S100A4 on the lymphocyte surface makes it impossible to form a complex with a target cell and to induce a subsequent cancer cell apoptosis.
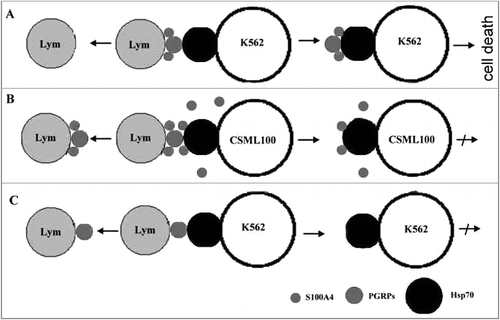
Hsp70 and S100A4 can form complexes with PGRPs and S100A4 that are present on the surfaces of CD4+CD25+PGRPs+S100A4+ lymphocytes if the former proteins are added one at a time (A). However, if Hsp70 and S100A4 are added together, S100A4 replaces Hsp70 from the complexes. This finding explains why cancer cells that synthesize and secret S100A4 are resistant to CD4+CD25+PGRPs+S100A4+ lymphocyte cytotoxicity. Specifically, S100A4 from the cancer cell's surface or from the intracellular medium interacts with PGRPs – S100A4 complex on the lymphocyte's membrane precluding that complex interaction with Hsp70 (B).
Figure 4. Interaction between CD4+CD25+PGRPs+S100A4+ lymphocytes and target cells in the presence and absence of S100A4 on their surface. А. S100A4 and PGRPs on the lymphocyte's surface form a complex with Hsp70 on the surface of target cell that do not express S100A4 during a lymphocyte-cell contact. B. S100A4 and PGRPs on the lymphocyte's surface do not form complex with Hsp70 on the surface of target cell that express S100A4 during a lymphocyte-cell contact. C. PGRPs on the lymphocyte's surface do not form complex with Hsp70 on the surface of target cell that do not express S100A4 during a lymphocyte-cell contact.
Further we confirmed that inhibitory mechanism by demonstrating that S100A4 expression level is positively correlated with the target cell's resistance to CD4+CD25+PGRPs+S100A4+ lymphocyte cytotoxicity. We provided an additional confirmation by demonstrating that S100A4 blockage by the antibodies restores the lymphocyte cytotoxicity.
The S100A4 ability to dissolve PGRPs – Hsp70 complex was previously established for liquid forms of those proteins. Specifically, the PGRP-S/Tag7- Hsp70 complex is secreted by CD3+CD8+ lymphocytes and is cytotoxic for various cancer cell lines [Citation17]. Using recombinant proteins, it was demonstrated that S100A4 precludes the interaction between PGRP-S and Hsp70 the way that a solvable complex loses its cytotoxic ability [Citation13].
In this study, the analyzed PGRPs – S100A4 complex is present on the surface of CD4+CD25+PGRPs+S100A4+ lymphocytes and the proteins are bound to the cells’ membranes. The presence of S100A4 on the lymphocytes’ membranes is required to form a stable cytotoxic complex, while the presence of S100A4 on the target cells’ membranes precludes the formation of that complex. S100A4 expression in target cells leads to S100A4 exposition on the lymphocytes’ membranes as a monomer and tetramer. This finding explains the absence of the cytotoxic reaction between the S100A4+ target cell and the lymphocyte. Such S100A4 expression on the target cells’ surface can be a potential way for a cancer cell to protect itself from the immune system defense.
We demonstrated that anticancer drug paclitaxel decreases the cytotoxic activity of interleukin-2 activated lymphocytes against К562 cell line. Paclitaxel caused a dose- and time-responsive inhibition of lymphocyte cytotoxicity. At the same time paclitaxel is not directly toxic to the lymphocytes [Citation14] but it significantly inhibits mts1 gene (S100A4) expression in cancer cells [Citation9]. We established that the decrease in cytotoxicity is associated with the absence of S100A4 on the lymphocytes’ membrane which is required for the contact lysis of К562 cells (C).
The addition of exogenous S100A4 in the incubation medium containing both paclitaxel-cultivated lymphocytes and target cells led to the decrease in the cytotoxicity but not lower than the 5% lethality level. We can propose that by adding the exogenous S100A4 we initiated two multidirectional processes: (1) S100A4 could interact with PGRPs on the lymphocytes’ membrane to form a cytotoxic complex and (2) S100A4 could replace Hsp70 from the complex on the target cells surface precluding the complete complex formation. The cytotoxicity was significantly restored then Са2+ was added to the medium. Са2+ was shown to catalyze the formation of PGRPs-S100A4 complex but not to interfere with the interaction between S100A4 and Hsp70. This illustrates that specifically the interaction of S100A4 with PGRPs increases the cytotoxicity.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Grigorian MS, Tulchinsky EM, Zain S, et al. The mts1 gene and control of tumor metastasis. Gene. 1993;135:229–238. DOI: 10.1016/0378-1119(93)90070-J PMID:8276262
- Rudland PS, Platt-Higgins A, Renshaw C, et al. Prognostic significance of the metastasis-inducing protein S100A4 (p9Ka) in human breast cancer. Cancer Res. 2000;60(6):1595–1603. PMID:10749128
- Takenaga K, Nakamura Y, Sakiyama S. Cellular localization of pEL98 protein, an S100-related calcium binding protein, in fibroblasts and its tissue distribution analyzed by monoclonal antibodies. Cell Struct Funct. 1994;3:133–141. DOI: 10.1247/csf.19.133.
- Dukhanina EA, Kabanova OD, Lukyanova TI, et al. Opposite roles of metastasin (S100A4) in two potentially tumoricidal mechanisms involving humanlymphocyte protein Tag7 and Hsp70. Proc Natl Acad Sci USA. 2009;106(33):13963–13967. DOI:10.1073/pnas.0900116106.
- Donato R, Cannon BR, Sorci G, et al. Functions of S100 Proteins. Curr Mol Med. 2013;13(1):24–57. DOI:10.1002/jemt.10296. PMID:22834835
- Dukhanina EA, Lukyanova TI, Romanova EA, et al. A new role for PGRP-S (Tag7) in immune defense: lymphocyte migration is induced by a chemoattractant complex of Tag7 with Mts1. Cell Cycle. 2015;14(22):3635–3643. DOI: 10.1080/15384101.2015.1104440. PMID:26654597
- Dahlmann M, Kobelt D, Walther W, et al. S100A4 in cancer metastasis: Wnt signaling-driven interventions for metastasis restriction. Cancers (Basel). 2016;8(6):1–22. DOI: 10.3390/cancers8060059.
- Dukhanina EA, Portseva TN, Pankratova EV, et al. Oct-1 modifies S100A4 exchange between intra- and extracellular compartments in Namalwa cells and increases their sensitivity to glucocorticoids. Cell Cycle. 2016;15(11):1471–1478. DOI: 10.1080/15384101.2016.1175260. PMID:27096393
- Cajone F, Debiasi S, Parker C, et al. Metastasis-associated mts1 gene expression is down-regulated by heat shock in variant cell lines of the B16 murine melanoma. Melanoma Res. 1994;4(3):143–150. DOI: 10.1089/dna.1994.13.343. PMID:7919958
- Weaver BA. How Taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677–2681. DOI: 10.1091/mbc.E14-04-0916. PMID:25213191
- Taylor S, Herrington S, Prime W, et al. S100A4 (p9Ka) protein in colon carcinoma and liver metastases: association with carcinoma cells and T-lymphocytes. Br J Cancer. 2002;86(3):409–416. DOI: 10.1038/sj.bjc.6600071. PMID:11875708
- Dukhanina EA, Lukyanova TI, Romanova EA, et al. Comparative Analysis of Secretion of S100A4 Metastatic Marker by Immune and Tumor Cells. Bull Exp Biol Med. 2008;145:85–87. DOI: 10.1007/s10517-008-0003-z.
- Dukhanina EA, Yashin DV, Galkin AV, et al. Unexpected deeds of familiar proteins: Interplay of Hsp70, PGRP-S/Tag7 and S100A4/Mts1 in host vs. cancer combat. Cell Cycle. 2010;9(4):676–682. DOI: 10.4161/cc.9.4.10782 PMID:20107319
- Chuang LT, Lotzová E, Heath J, et al. Alteration of lymphocyte microtubule assembly, cytotoxicity, and activation by the anticancer drug taxol. Cancer Res. 1994;54(5):1286–1291. PMID:7907000
- Dukhanina EA, Romanova EA, Dukhanin AS, et al. Interactions and possible functional characteristics of Tag7-S100A4 protein complex. Bull Exp Biol Med. 2008;145(2):191–193. DOI: 10.1007/s10517-008-0047-0.
- Ebralidze A, Tulchinsky E, Grigorian M, et al. Isolation and characterization of a gene specifically expressed in different metastatic cells and whose deduced gene product has a high degree of homology to a Ca2+-binding protein family. Genes Dev. 1989;3(7):1086–1093. DOI: 10.1101/gad.3.7.1086. PMID:2550322
- Sashchenko LP, Dukhanina EA, Yashin DV, et al. Peptidoglycan recognition protein tag7 forms a cytotoxic complex with heat shock protein 70 in solution and in lymphocytes. J Biol Chem. 2004;279(3):2117–2124. DOI:10.1074/jbc.M307513200. PMID:14585845
