ABSTRACT
Gastrointestinal stromal tumor (GIST) with a mutation in exons 11 and 17 of c-kit is a rare type of sarcoma. The aim of this study was to determine drug sensitivity for a regionally-recurrent case of GIST using a patient-derived orthotopic xenograft (PDOX) model. The PDOX model was established in the anterior wall of the stomach. GIST PDOX models were randomized into 5 groups of 6 mice each when the tumor volume reached 60 mm3: G1, control group; G2, imatinib group (oral administration (p.o.), daily, for 3 weeks); G3, sunitinib group (p.o., daily, for 3 weeks); G4, regorafenib (p.o., daily, for 3 weeks); G5, pazopanib (p.o., daily, for 3 weeks). All mice were sacrificed on day 22. Tumor volume was evaluated on day 0 and day 22 by laparotomy. Body weight were measured 2 times per week. Though regorafenib is third-line therapy for GIST, it was the most effective drug and regressed the tumor significantly (p < 0.001). Sunitinib suppressed tumor growth compared to the control group (p = 0.002). Imatinib, first-line therapy for GIST, and pazopanib did not have significant efficacy compared to the control group (p = 0.886, p = 0.766). The implications of this result is discussed for GIST patients.
Introduction
Gastrointestinal stromal tumor (GIST) is a rare type of sarcoma derived from the muscle layer of the gastrointestinal tract [Citation1]. The majority of GIST tumors have a mutation in the tyrosine kinase receptor c-kit [Citation2] in exons 11 (70–80%) and exons 9 (10%) [Citation2,Citation3]. GIST tumors without a c-kit mutation may have a platelet-derived growth factor receptor α (PDGFRα) mutation, similar to the c-kit gene, in about 10% of the cancers usually in exon 18 (10%) of the c-kit gene [Citation3]. The majority of the PDGFRα mutations occured in exons 18 (10%) [Citation3]. Recently mutations in the BRAF gene and a gene encoding the protein succinate dehydrogenase (SDH) have been observed in GIST without c-kit gene and PDGFRα mutations [Citation4].
Complete R0 surgical resection without tumor rupture and spillage is the main curative therapy for GIST [Citation5]. Not all cases, however, are resectable due to tumor size and distant metastases or patient characteristics. Usually, recurrent cases are also inoperable. Tyrosine kinase inhibitors are indicated for inoperable cases with imatinib (Gleevec) as first-line therapy as described in the American National Comprehensive Cancer Network (NCCN) guidelines [Citation6]. Imatinib was originally developed as a molecular-targeting drug for chronic myelogenous leukemia (CML) [Citation7], and subsequently approved for GIST by the US Food and Drug Administration (FDA) in 2001 [Citation8]. Sunitinib and regorafenib are multi-kinase inhibitors which are used as second- and third-line treatment, respectively [Citation9,Citation10]. However, the NCCN guidelines are imprecise due to the individual characteristics of each tumor [Citation11].
The aim of the present study was to identify the power of the PDOX model to identify effective therapy for recalcitrant cancer, in this case, Imitanib-resistant recurrent GIST. Toward this goal of precision personalized oncology, our laboratory pioneered the patient-derived orthotopic xenograft (PDOX) nude mouse model with the technique of surgical orthotopic implantation (SOI), including pancreatic [Citation12–15], breast [Citation16], ovarian [Citation17], lung [Citation18], cervical [Citation19], colon [Citation20–22], stomach [Citation23], sarcoma [Citation24–38] and melanoma [Citation39–43].
In the present study, we established a PDOX model for a recurrent GIST patient and evaluated the efficacy of first, second and third-line therapy.
Results and discussion
The GIST PDOX model was established in the gastric wall by orthotopic implantation in nude mice (). shows the treatment schema for the GIST PDOX model. Regorafenib regressed the GIST tumor compared to the un-treated group (P<0.001). Sunitinib suppressed, but not regressed, tumor growth compared to the un-treated group (P = 0.002). Imatinib and pazopanib did not show significant efficacy compared to the un-treated group (P = 0.886 and P = 0.766). The final tumor volume ratio (post-treatment tumor volume / pre-treatment tumor volume) on day 22 was as follows: untreated control group (G1) 4.91±1.55; imatinib group (G2) 4.30±0.80; sunitinib group (G3) 2.06±0.31; regorafenib group (G4) 0.15±0.05; pazopanib group (G5) 4.14±1.50 ().
Figure 1. Surgical orthotopic implantation (SOI). A. An approximately 10 mm incision was performed at the upper portion of the abdomen (black dotted line). B. The stomach was gently exteriorized from the peritoneum. C. The serosa of the anterior gastric wall was carefully incised in order to not make a perforation (white arrow). D. A small fragment of GIST was implanted at the incised site on the gastric wall (white arrow).
Figure 2. Treatment protocol. G1: untreated group; G2: imatinib (50 mg/kg, p.o., daily, 3 weeks); G3: sunitinib (40 mg/kg, p.o., daily, 3 weeks); G4: regorafenib (30 mg/kg, p.o., daily, 3 weeks); G5: pazopanib (100 mg/kg, p.o., daily, 3 weeks). Each group consisted of 6 mice. All mice were sacrificed on day 22.
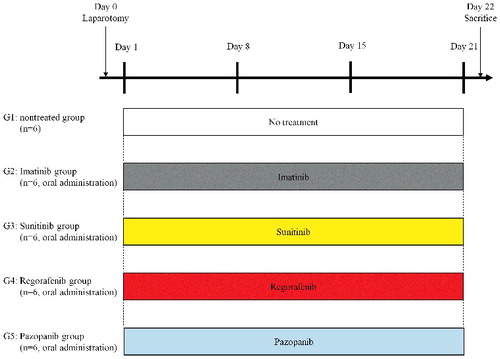
Figure 3. Tumor volume ratio. Bar graphs show the PDOX tumor volume ratio (post-treatment volume / pre-treatment volume) on day 0 and 22. Regorafenib regressed tumor growth significantly compared to the untreated group (P < 0.001). There was also a significant difference between the untreated group and sunitinib (P = 0.002). Imatinib and pazopanib did not show significant efficacy (P = 0.886 and P = 0.766) respectively compared to the untreated control. Error bars: ± SD.
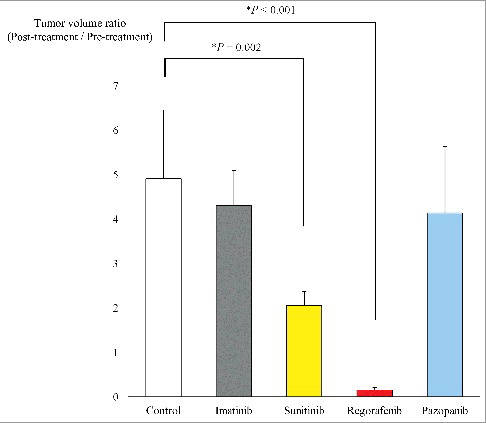
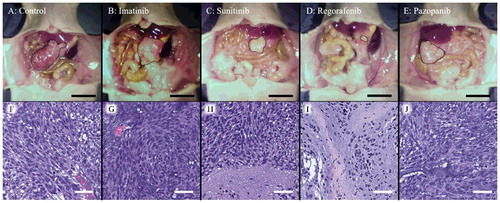
Though the body weight of the regorafenib group slightly decreased, there was no significant difference in body weight on day 0 and day 22 between the five groups (). shows representative tumors from each group at laparotomy on day 22 as well as photomicrographs of hematoxylin and eosin (H&E)-stained sections of a tumor from each group. Necrosis due to chemotherapy was found in the sunitinib and regorafenib groups ( H and I).
Figure 4. Body weight of each group. Bar graphs shows body weight of the GIST PDOX mice treated with each drug. There was no significant difference between any group. Error bars: ± SD.
Figure 5. A-E. Representative macroscopic images of the GIST PDOX model. Representative images of the GIST PDOX on day 22. The area surrounded by the black broken line is tumor. Scale bar is 10 mm. F-J. Hematoxylin and eosin (H&E) staining of a representative tumor from each group. Sunitinib and regorafenib showed necrosis (H and I). Scale bar is 50 µm.
In the present study, only regorafenib, which is a third line drug in the NCCN guidelines, regressed tumor growth in the GIST PDOX model. Regorafenib is an oral multi-kinase inhibitor, and was approved for metastatic colorectal cancer by the FDA in 2012 [Citation44] and for Advanced GIST in 2013 [Citation10]. A multi-center Phase II clinical trial showed efficacy of regorafenib as third-line therapy in metastatic or unresectable GIST patients after failure of imatinib and sunitinib [Citation45]. The role of regorafenib as a first and second line therapy is currently being studied in clinical trials (NCT02365441 and NCT02164240).
Previously-developed concepts and strategies of highly-selective tumor targeting can take advantage of molecular targeting of tumors, including tissue-selective therapy which focuses on unique differences between normal and tumor tissues [Citation46–51].
Conclusions
Regorafenib regressed recurrent imatanib-resistant GIST in a PDOX model. The GIST PDOX model should enable precise, ndividualized, improved therapy for patients with this disease.
Materials and methods
Animals
Athymic nu/nu male nude mice (AntiCancer, Inc., San Diego, CA), 4–6 weeks old, were used in this study. All mice were kept in a barrier facility on a high-efficiency particulate arrestance (HEPA)-filtered rack under standard conditions of 12-hour light/dark cycles. The animals were fed an autoclaved laboratory rodent diet [Citation27]. All animal experiments were performed with an AntiCancer Institutional Animal Care and Use Committee (IACUC)-protocol specifically approved for this study and in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals under Assurance Number A3873-1. Anesthesia and analgesics were used for all surgical experiments to avoid unnecessary suffering of the mice. Subcutaneous injection of a ketamine mixture (a 0.02 ml solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate) was used for mice. The response of animals during surgery was monitored carefully to keep adequate depth of anesthesia. The animals were observed daily and humanely sacrificed by CO2 inhalation when they met the following criteria: severe tumor burden (more than 20 mm in diameter), prostration, significant body weight loss, difficulty breathing, rotational motion and body temperature drop.
Establishment of the GIST PDOX model
The patient received curative-intent surgery in the Department of Surgery, University of California, Los Angeles (UCLA). The GIST recurred regionally. The recurrent site was resected after neoadjuvant chemotherapy with imatinib. The tumor had c-kit exon 11 and 17 mutations. Resected fresh tumor was brought to AntiCancer Inc. from the UCLA Hospital and established in nude mice. Informed consent was previously obtained from the patient, and this study was approved by the Institutional Review Board of UCLA (IRB #10-001857). The GIST PDOX was established by orthotopic implantation to the anterior gastric wall ().
Treatment protocol for the GIST PDOX model
The PDOX models were randomized into 5 groups when tumor volume reached 60 mm3; G1: untreated control group; G2: imatinib (50 mg/kg, p.o., daily, 3 weeks); G3: sunitinib (40 mg/kg, p.o., daily, 3 weeks); G4: regorafenib (30 mg/kg, p.o., daily, 3 weeks); G5: pazopanib (100 mg/kg, p.o., daily, 3 weeks) (). Doses of the above drugs were determined by published reports (29–32). Tumor volume was evaluated on day 0 and day 22 by laparotomy using the following formula: tumor volume (mm3) = length (mm) × width (mm) × width (mm) × 1/2. Body weight was measured 2 times a week. All cases were sacrificed on day 22.
Histological examination
10% formalin-fixed, paraffin-embedded tissue sections (5 μm) were deparaffinized in xylene and rehydrated in an ethanol series. Hematoxylin and eosin (H&E) staining was performed according to a standard protocol. Histological examination was observed with a BHS system microscope (Olympus Corp., Tokyo, Japan).
Statistical analysis
All statistical analyses were performed with Statistical Package for the Social Sciences for Windows software version 22.0 (IBM Corp., Armonk, NY, USA). Significant differences for comparisons of ≥ 3 groups were determined using one-way ANOVA followed by Tukey post hoc pairwise tests. Bar graphs show the mean, and error bars express ± standard deviations. A probability value of P < 0.05 was defined as statistically significant.
Dedication
This paper is dedicated to the memory of A. R. Moossa, M.D., and Sun Lee, M.D.
Disclosure of potential conflicts of interest
K.M., K.K., T.K., M.M., I.K., Z.Z., T.M., and R.M.H. are unsalaried associates of AntiCancer Inc. There are no other competing financial interests.
Acknowledgments
This study was supported in part by the National Cancer Institute grant CA 213649.
References
- Miettinen M, Lasota J. Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archiv. 2001;438:1–12. doi:10.1007/s004280000338. PMID:11213830
- Tornillo L, Terracciano LM. An update on molecular genetics of gastrointestinal stromal tumours. J Clin Path. 2006;59:557–563. doi:10.1136/jcp.2005.031112. PMID:16731599
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi:10.1126/science.279.5350.577. PMID:9438854
- Agaimy A, Terracciano LM, Dirnhofer S, et al. V600E BRAF mutations are alternative early molecular events in a subset of KIT/PDGFRA wild-type gastrointestinal stromal tumours. J Clin Path. 2009;62:613–616. doi:10.1136/jcp.2009.064550. PMID:19561230
- Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3–14. doi:10.1007/s10120-015-0526-8. PMID:26276366
- Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1–41. doi:10.6004/jnccn.2010.0116. PMID:20457867
- Habeck M. FDA licences imatinib mesylate for CML. Lancet Oncol. 2002;3:6. doi:10.1016/S1470-2045(01)00608-8. PMID:11905608
- Dagher R, Cohen M, Williams G, et al. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res. 2002;8:3034–3038. PMID:12374669
- Goodman VL, Rock EP, Dagher R, et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res. 2007;13:1367–1373. doi:10.1158/1078-0432.CCR-06-2328. PMID:17332278
- FDA approves regorafenib (Stivarga) for GIST. Oncol (Williston Park, NY). 2013;27:164.
- Demetri GD, Benjamin RS, Blanke CD, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)–update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5 Suppl 2:S1–29. PMID:17624289
- Hiroshima Y, Zhang Y, Murakami T, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograph (PDOX) and cell line mouse models. Oncotarget. 2014;5:12346–12357.
- Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci USA. 1992;89:5645–5649. doi:10.1073/pnas.89.12.5645. PMID:1608975
- Hiroshima Y, Maawy A, Zhang Y, et al. Metastatic recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) nude mouse model is inhibited by neoadjuvant chemotherapy in combination with fluorescence-guided surgery with an anti-CA 19–9-conjugated fluorophore. PLoS One. 2014;9:e114310. doi:10.1371/journal.pone.0114310. PMID:25463150
- Hiroshima Y, Maawy AA, Katz MH, et al. Selective efficacy of zoledronic acid on metastasis in a patient-derived orthotopic xenograph (PDOX) nude-mouse model of human pancreatic cancer. J Surg Oncol. 2015;111:311–315. doi:10.1002/jso.23816. PMID:25394368
- Fu X, Le P, Hoffma RM. A metastatic-orthotopic transplant nude-mouse model of human patient breast cancer. Anticancer Res. 1993;13:901–904. PMID:8352558
- Fu X, Hoffman RM. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res. 1993;13:283–286. PMID:8517640
- Wang X, Fu X, Hoffman RM. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int J Cancer. 1992;51:992–995. doi:10.1002/ijc.2910510626. PMID:1639545
- Hiroshima Y, Zhang Y, Zhang N, et al. Establishment of a patient-derived orthotopic xenograph (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLoS One. 2015;10:e0117417. doi:10.1371/journal.pone.0117417. PMID:25689852
- Fu X, Besterman JM, Monosov A, et al. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci USA. 1991;88:9345–9349. doi:10.1073/pnas.88.20.9345. PMID:1924398
- Metildi CA, Kaushal S, Luiken GA, et al. Fluorescently-labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Surg Oncol. 2014;109:451–458. doi:10.1002/jso.23507. PMID:24249594
- Hiroshima Y, Maawy A, Metildi CA, et al. Successful fluorescence-guided surgery on human colon cancer patient-derived orthotopic xenograft mouse models using a fluorophore-conjugated anti-CEA antibody and a portable imaging system. J Laparoendosc Adv Surg Tech A. 2014;24:241–247. doi:10.1089/lap.2013.0418. PMID:24494971
- Furukawa T, Kubota T, Watanabe M, et al. Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: correlation of metastatic sites in mouse and individual patient donors. Int J Cancer. 1993;53:608–612. doi:10.1002/ijc.2910530414. PMID:8436434
- Murakami T, DeLong J, Eilber FC, et al. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft PDOX model. Oncotarget. 2016;7:12783–12790.
- Hiroshima Y, Zhao M, Zhang Y, et al. Tumor-targeting Salmonella typhimurium A1-R arrests a chemo-resistant patient soft-tissue sarcoma in nude mice. PLoS One. 2015;10:e0134324. doi:10.1371/journal.pone.0134324. PMID:26237416
- Kiyuna T, Murakami T, Tome Y, et al. High efficacy of tumor-targeting Salmonella typhimurium A1-R on a doxorubicin- and dactolisib-resistant follicular dendritic-cell sarcoma in a patient-derived orthotopic xenograft PDOX nude mouse model. Oncotarget. 2016;7:33046–33054. doi:10.18632/oncotarget.8848. PMID:27105519
- Murakami T, Singh AS, Kiyuna T, et al. Effective molecular targeting of CDK4/6 and IGF-1R in a rare FUS-ERG fusion CDKN2A-deletion doxorubicin-resistant Ewing's sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2016;7:47556–47564.
- Hiroshima Y, Zhang Y, Zhang N, et al. Patient-derived orthotopic xenograft (PDOX) nude mouse model of soft-tissue sarcoma more closely mimics the patient behavior in contrast to the subcutaneous ectopic model. Anticancer Res. 2015;35:697–701. PMID:25667448
- Igarashi K, Murakami T, Kawaguchi K, et al A patient-derived orthotopic xenograft (PDOX) mouse model of an cisplatinum-resistant osteosarcoma lung metastasis that was sensitive to temozolomide and trabectedin: implications for precision oncology. Oncotarget. 2017;8:62111–62119. PMID:28977930
- Igarashi K, Kawaguchi K, Kiyuna T, et al. Temozolomide combined with irinotecan caused regression in an adult pleomorphic rhabdomyosarcoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2017;8:75874–75880. PMID:29100276
- Igarashi K, Kawaguchi K, Murakami T, et al. Intra-arterial administration of tumor-targeting Salmonella typhimurium A1-R regresses a cisplatin-resistant relapsed osteosarcoma in a patient-derived orthotopic xenograft (PDOX) mouse model. Cell Cycle. 2017;16:1164–1170. doi:10.1080/15384101.2017.1317417. PMID:28494180
- Murakami T, Kiyuna T, Kawaguchi K, et al. The irony of highly-effective bacterial therapy of a patient-derived orthotopic xenograft (PDOX) model of Ewing's sarcoma, which was blocked by Ewing himself 80 years ago. Cell Cycle. 2017;16:1046–1052. doi:10.1080/15384101.2017.1304340. PMID:28296559
- Igarashi K, Kawaguchi K, Murakami T, et al. High efficacy of pazopanib on an undifferentiated spindle-cell sarcoma resistant to first-line therapy is identified with a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Cell Biochem. 2017;118:2739–3743. doi:10.1002/jcb.25923. PMID:28176365
- Kiyuna T, Murakami T, Tome Y, et al. Analysis of stroma labeling during multiple passage of a sarcoma imageable patient-derived orthotopic xenograft (iPDOX) in red fluorescent protein transgenic nude mice. J Cell Biochem. 2017;118:3367–3371. doi:10.1002/jcb.25991. PMID:28300287
- Igarashi K, Murakami T, Kawaguchi K, et al. A patient-derived orthotopic xenograft (PDOX) mouse model of an cisplatinum-resistant osteosarcoma lung metastasis that was sensitive to temozolomide and trabectedin: implications for precision oncology. Oncotarget. 2017;8:62111–62119. PMID:28977930
- Igarashi K, Kawaguchi K, Kiyuna T, et al. Temozolomide combined with irinotecan caused regression in an adult pleomorphic rhabdomyosarcoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2017;8:75874–75880. PMID:29100276
- Igarashi K, Kawaguchi K, Murakami T, et al. A novel anionic-phosphate-platinum complex effectively targets an undifferentiated pleomorphic sarcoma better than cisplatinum and doxorubicin in a patient-derived orthotopic xenograft (PDOX). Oncotarget. 2017;8:63353–63359. PMID:28968995
- Miyake K, Murakami T, Kiyuna T, et al. The combination of temozolomide-irinotecan regresses a doxorubicin-resistant patient-derived orthotopic xenograft (PDOX) nude-mouse model of recurrent Ewing's sarcoma with a FUS-ERG fusion and CDKN2A deletion: Direction for third-line patient therapy. Oncotarget. 2017;8:103129–103136. PMID:29262551
- Kawaguchi K, Murakami T, Chmielowski B, et al. Vemurafenib-resistant BRAF-V600E mutated melanoma is regressed by MEK targeting drug trametinib, but not cobimetinib in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget. 2016;7:71737–71743. PMID:27690220
- Kawaguchi K, Igarashi K, Murakami T, et al. Tumor-targeting Salmonella typhimurium A1-R combined with temozolomide regresses malignant melanoma with a BRAF-V600 mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget. 2016;7:85929–85936.
- Kawaguchi K, Igarashi K, Murakami T, et al. Tumor-targeting Salmonella typhimurium A1-R sensitizes melanoma with a BRAF-V600E mutation to vemurafenib in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Cell Biochem. 2017;118:2314–2319. doi:10.1002/jcb.25886. PMID:28106277
- Kawaguchi K, Igarashi K, Li S, et al. Combination treatment with recombinant methioninase enables temozolomide to arrest a BRAF V600E melanoma growth in a patient-derived orthotopic xenograft. Oncotarget. 2017;8:85516–85525. PMID:29156737
- Yamamoto M, Zhao M, Hiroshima Y, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R on a melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. PLoS One. 2016;11:e0160882. doi:10.1371/journal.pone.0160882. PMID:27500926
- FDA approves regorafenib (Stivarga) for metastatic colorectal cancer. Oncol (Williston Park, NY). 2012;26:896.
- Ben-Ami E, Barysauskas CM, von Mehren M, et al. Long-term follow-up results of the multicenter phase II trial of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of standard tyrosine kinase inhibitor therapy. Ann Oncol. 2016;27:1794–1799. doi:10.1093/annonc/mdw228. PMID:27371698
- Blagosklonny MV. Matching targets for selective cancer therapy. Drug Discov Today. 2003;8:1104–1107. doi:10.1016/S1359-6446(03)02806-X. PMID:14678733
- Blagosklonny MV. Teratogens as anti-cancer drugs. Cell Cycle. 2005;4:1518–1521. doi:10.4161/cc.4.11.2208. PMID:16258270
- Blagosklonny MV. Treatment with inhibitors of caspases, that are substrates of drug transporters, selectively permits chemotherapy-induced apoptosis in multidrug-resistant cells but protects normal cells. Leukemia. 2001;15:936–941.
- Blagosklonny MV. Target for cancer therapy: proliferating cells or stem cells. Leukemia. 2006;20:385–391. doi:10.1038/sj.leu.2404075. PMID:16357832
- Apontes P, Leontieva OV, Demidenko ZN, et al. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget. 2011;2:222–233. doi:10.18632/oncotarget.248. PMID:21447859
- Blagosklonny MV. Tissue-selective therapy of cancer. Br J Cancer. 2003;89:1147–1151. doi:10.1038/sj.bjc.6601256. PMID:14520435
