ABSTRACT
The mechanisms behind the Warburg effect in mammalian cells, as well as for the similar Crabtree effect in the yeast Saccharomyces cerevisiae, are still a matter of debate: why do cells shift from the energy-efficient respiration to the energy-inefficient fermentation at high sugar concentration?
This review reports on the strong similarities of these phenomena in both cell types, discusses the current ideas, and provides a novel interpretation of their common functional mechanism in a dynamic perspective. This is achieved by analysing another phenomenon, the sugar-induced-cell-death (SICD) occurring in yeast at high sugar concentration, to highlight the link between ATP depletion and cell death.
The integration between SICD and the dynamic functioning of the glycolytic process, suggests that the Crabtree/Warburg effect may be interpreted as the avoidance of ATP depletion in those conditions where glucose uptake is higher than the downstream processing capability of the second phase of glycolysis. It follows that the down-regulation of respiration is strategic for cell survival allowing the allocation of more resources to the fermentation pathway, thus maintaining the cell energetic homeostasis.
1. Introduction
It is not surprising that fermentation is maintained in most cells, allowing their proliferation also in anoxic environments. Not so obvious, instead, is the occurrence of fermentation in aerobic conditions, that is the predominance of a fermentative metabolism at high glucose concentration even with available oxygen.
Aerobic fermentation leading to lactate in proliferating mammalian cells is commonly known as Warburg effect, a tribute to Otto Warburg, who was the first to observe it in the 1920s [Citation1–4]. This phenomenon has also been extensively studied in the yeast Saccharomyces cerevisiae, the unicellular model eukaryote, and is commonly referred to as Crabtree effect. The term derives from the early observations by Herbert Crabtree in his studies on tumour cells [Citation5] and then extended to yeasts [Citation6]. Indeed, the metabolic shift from an energy-efficient to an energy-inefficient pathway with the apparent spilling of energy resources is not restricted to yeast and (tumour and non-tumour) mammalian cells [Citation7–11], since it has been also observed in many unicellular organisms growing at high substrate concentration [Citation12].
Furthermore, the Warburg effect is considered a hallmark of cancer development and for this reason, nowadays, is subjected to very intensive investigations and focus of attention [Citation13].
In this review, considering the strong similarities of aerobic fermentation in both yeast and mammalian cells [Citation9,Citation10], we will use the term Crabtree/Warburg effect to indicate the aerobic fermentation in general, whereas Crabtree effect and Warburg effect alone will indicate the phenomenon exclusively in the case of yeast or mammalian cells, respectively. Instead, we consider misleading the terms “aerobic glycolysis” and “glycolytic cells”, often reported in the literature to indicate aerobic fermentation and fermentative cells in aerobic conditions, respectively. Indeed, the glycolytic pathway is shared by both fermentation and respiration and so, it cannot be exclusively associated to the Crabtree/Warburg effect. The latter, indeed, can only be related to the enhanced glycolytic flux, as we will discuss below.
During aerobic fermentation, the occurrence of active mechanisms to reduce or even suppress respiration in favour of a less efficient catabolic pathway, suggests the existence of a fitness advantage for the cell [Citation14]. However, notwithstanding the intense interest about the Crabtree/Warburg effect, a clear explanation of the benefits associated to this phenomenon still remains elusive [Citation15,Citation16]. Why is the energy-inefficient fermentation pathway preferred to the more energy-efficient respiration process even in conditions suitable for the latter? Which fitness advantage does it provide?
In this paper, we discuss the several proposals emerged over the years (reviewed by [Citation12,Citation14–16]) to explain the apparent paradox [Citation17] of cells switching to a less efficient metabolism when exposed to high sugar concentration.
In yeast, another phenomenon occurring at high sugar concentration which may seem unrelated to the Crabtree effect is the so-called sugar-induced cell death (SICD), which refers to the rapid loss of viability of stationary cells of S. cerevisiae when transferred to a medium containing only glucose [Citation18–21].
Here, by a punctual analysis of the cases of SICD reported in the literature, we highlight the link between ATP depletion and cell death; then we propose a novel interpretation of the Crabtree/Warburg effect based on the integration between the phenomenon of SICD and the functioning of the glycolytic process.
Logical reasoning on the dynamics of the glycolytic pathway shows the relevance of the different rates of the glycolytic reactions in relation to the ATP homeostasis of the cell and provides an interpretation of the Crabtree/Warburg effect as a fitness advantage in terms of a basic survival strategy for cells.
2. Respiration and fermentation in yeast and mammalian cells
Starting from glucose, the two energetically different pathways of respiration and fermentation in Saccharomyces cerevisiae and mammalian cells, are schematically depicted in . Both fermentation and respiration start with glycolysis (from glucose to pyruvate), which yields 2 ATP and 2 NADH moles per mole of glucose in all cell types. In the yeast fermentative pathway, pyruvate is transformed to acetaldehyde by pyruvate decarboxylase (Pdc) and then acetaldehyde to ethanol by alcohol dehydrogenase (Adh), resulting in the re-oxidation of NADH to NAD+. In mammalian cells, pyruvate is directly fermented to lactate by lactate dehydrogenase (Ldh), and concomitantly NADH is re-oxidized to NAD+, with the same ATP yield per glucose mole as in yeast.
Figure 1. Schematic representation of respiration and fermentation pathways and related ATP production in yeast and mammalian cells. Pdc, pyruvate decarboxylase; Pdh, pyruvate dehydrogenase; Aldh, acetaldehyde dehydrogenase; Acs, acetyl-CoA synthetase; Adh, alcohol dehydrogenase; Ldh, lactate dehydrogenase. Adh1 and Adh2, Ldh1 and Ldh2 indicate different isoforms of alcohol dehydrogenase and lactate dehydrogenase, respectively.
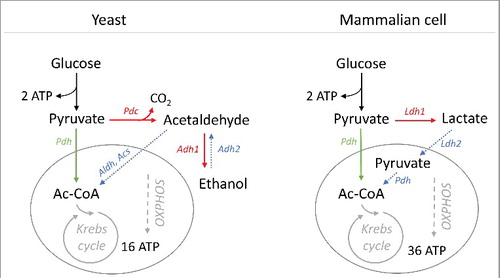
In both cell types, respiration is compartmentalized in the mitochondrion, and consists in the conversion of pyruvate into acetyl-CoA by pyruvate dehydrogenase (Pdh), followed by a complete oxidation to CO2 in the Krebs cycle; NADH produced during glycolysis is re-oxidized by oxidative phosphorylation (OXPHOS) in the mitochondrion, where ATP is formed. Overall, the ATP gain of respiration is 18 and 36 ATP moles per mole of glucose in S. cerevisiae [Citation22] and mammalian cells [Citation23], respectively. The difference in ATP efficiency between fermentative and respiratory metabolism also leads to different biomass yields: in yeast, it has been ascertained that five-fold increase in biomass yield is achieved by respiration [Citation24].
When glucose is abundant, fermentation is the main catabolic pathway even in the presence of oxygen (Crabtree/Warburg effect). Some yeast species do not display a significant Crabtree effect and are commonly designated as Crabtree-negative yeasts, their metabolism being predominantly respiratory, even in the presence of high glucose concentration [Citation25,Citation26].
In yeast metabolism () the ethanol produced by fermentation, once glucose is depleted, can be recycled and respired in the mitochondrion via acetyl-CoA [Citation27]. Similarly, the lactate produced by mammalian cells, transiently excluded from the cell, can be afterwards gradually taken up and consumed by respiration () [Citation28–31]. In such latter process, the occurrence of monocarboxylate transporters (MCTs) in the inner mitochondrial membrane, and a specific isoform of Ldh ensures the direct conversion of lactate into the mitochondrial pool of pyruvate [Citation32,Citation33]. Differently from yeast, some cancer cells, producing lactate in presence of high glucose concentration, are concomitantly able to respire it [Citation29,Citation30], thus showing a metabolic plasticity crucial for their adaptive success.
3. Warburg effect in cancer cells
The fermentation of glucose to lactate (the Warburg effect) was the first biochemical trait assigned to cancer already in the 1920's. Warburg assumed that mitochondria were not functional in cancer cells, but this idea has been largely questioned because some tumour cell line have been reported to display oxidative metabolism [Citation34] with many studies indicating that also mitochondrial activity and oxidative phosphorylation may support tumour growth [Citation35,Citation36].
Tumour consists of a complex milieu of malignant and non-malignant cell types with distinct metabolic features, differential consumption of nutrients and symbiotic relationship among cells [Citation37–39]. It has been shown that, besides glucose, a panoply of fuels (glutamine, fatty acids and lactate) can be utilized by tumour cells, thus contributing to their metabolic function and plasticity [Citation40]). As lactate recycling is concerned, it has been shown that oxidative tumour cells (near the blood vessels) are able to consume the lactate secreted by tumour cells which perform aerobic glycolysis [Citation41]. A so-called “reverse Warburg effect” has been also described with respect to the metabolic interplay between glycolytic stromal cells surrounding a tumour and oxidative tumour cells, with the former cells feeding the tumour with lactate [Citation42]. Moreover, fibroblasts surrounding a tumour can exchange nutrients and signals via exosomes with the adjacent tumour cells, surprisingly inhibiting their oxidative phosphorylation [Citation43].
Very recently, it has been shown that normal fibroblasts of individuals born with inherited mutation in BAP1 (BAP1+/−), (BAP1 is a deubiquitylase BRCA1-associated protein) display a typical Warburg effect, with enhanced glycolysis, increased lactate production and reduced mitochondrial respiration, compared with BAP1wt cells [Citation44]. Since BAP1+/− mutation has a critical relevance for cancer predisposition, the originality of this finding relies on the fact that the Warburg effect might facilitate cancer transformation rather than being an adaptive process following malignancy [Citation45].
Indeed, the Warburg effect has been traditionally associated to uncontrolled cell proliferation. Instead, it is worth remarking that not all proliferative cells use aerobic glycolysis [Citation46] as already mentioned in the case of some tumour cells displaying oxidative metabolism. Conversely, some non-dividing cells such as quiescent human fibroblasts [Citation47], as well as anoxic hematopoietic stem cells [Citation48], preferentially rely on glucose fermentation to generate ATP.
High proliferating cells (both tumour and non-tumour), described as Warburg positive, are usually characterized by high rates of glycolysis, lactate production and also increased macromolecular biosynthesis [Citation7,Citation8]. In this context, the proliferative “advantage” of the Warburg effect, should consist in the ability to provide fast ATP production as well as the building blocks for biosynthesis. As discussed later in this review (see Section 5), we argue that there is no causal relationship between metabolic activity (either fermentation or respiration) and the proliferative status of the cell. The often-reported observation of correlation between Warburg and proliferation is, rather, a consequence of the conditions of high nutrient availability.
4. Short- and long-term Crabtree/Warburg effects
In S. cerevisiae, a short-term Crabtree effect has been clearly distinguished from a long-term one (). According to Pronk et al. [Citation49], the short-term Crabtree effect is defined as the immediate onset of alcoholic fermentation upon addition of excess sugar to sugar-limited respiratory yeast cultures. This phenomenon has been well explained as an “overflow” in glucose metabolism, which outpaces the maximal velocity of pyruvate oxidation, the latter caused by a limited respiratory capacity due to physical and/or biochemical constraints (B) [Citation49–51]. So, the number of mitochondria and/or the mitochondrion capacity itself may constitute a “bottleneck” for respiration which soon becomes evident when sugar concentration is high. Moreover, the different kinetic properties of the two main enzymes of fermentation and respiration, pyruvate decarboxylase (Pdc) and pyruvate dehydrogenase (Pdh) respectively, have to be considered: at low pyruvate concentrations, respiration is favored due to the higher affinity of Pdh towards pyruvate; contrarily, at high pyruvate concentrations, fermentation predominates since the Vmax of Pdc is higher than that of Pdh [Citation49].
Figure 2. Simplified representation of carbon flux and mitochondrial “bottleneck” at different levels of glucose concentration: (A) respiration; (B) short- and (C) long-term Crabtree/Warburg effects. The key enzymes involved in pyruvate handling are indicated: Pdh (pyruvate dehydrogenase), Pdc (pyruvate decarboxylase) in yeast, and Ldh1 (lactate dehydrogenase) in mammalian cells.
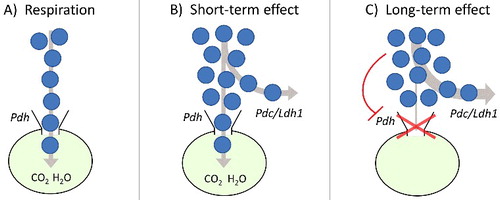
On the other hand, the long-term Crabtree effect, which is defined as the aerobic alcoholic fermentation which establishes under steady-state conditions at high glucose concentration [Citation27], is reported to be related to repression of genes involved in oxidative metabolism, and linked to the composite phenomenon of glucose repression [Citation52,Citation53]. The latter involves the down-regulation of oxidative metabolism related proteins (including Pdh), in addition to the enhanced transcription of glucose transporters and glycolytic enzymes. Further, accumulation of fructose-1,6-biphoshate has been shown to induce a decrease in the activity of mitochondrial complexes III and IV in both yeast and rat liver isolated mitochondria [Citation54]. Several models of yeast growth have been developed since 1980s considering the “bottleneck/overflow” concept and the glucose repression phenomena [Citation55–57]. Recently, a system dynamics model of yeast growth well reproduced the occurrence of both the “bottleneck/overflow” (short-term) and the glucose repression (long-term) Crabtree effect as a function of different glucose feeding conditions [Citation58].
The distinction between a short and a long-term effect has been also reported in fermentative proliferating mammalian cells [Citation9,Citation10]. In this case, short-term indicates the reversible shift from respiration to fermentation and it is referred to as “Crabtree” effect, while the long-term metabolic re-programming is named tout court Warburg effect, thus considering the Crabtree effect as an early event, leading to the establishment of the Warburg effect. The same literature also claims that the short-term effect in both tumour cells and yeast is determined by the same mechanisms: competition for ADP and Pi between glycolysis and mitochondria, reduced permeability of the mitochondrial outer membrane, role of Ca2+ in decreasing respiration, and fructose-1,6-diphosphate mediating down-regulation of respiration. It is worth noting that the reduced permeability of the mitochondrial membrane observed in tumour cells indeed may correspond to the “bottleneck” responsible for the short-term effect in yeast. Instead, the role of fructose-1,6-diphosphate levels down-regulating respiration may be considered as a long-term effect.
Interestingly, Curi et al. [Citation59] reported that in proliferative mammalian cells, the glycolytic flux may exceed the Vmax of Pdh by more than one order of magnitude, which can be seen as further evidence of the respiration “bottleneck”. The authors speculate that to avoid accumulation of pyruvate, the cell rapidly starts producing lactate that can be easily secreted by an Ldh over-expressing cell.
In conclusion, short- and long-term effects have been recognized in both yeast and mammalian cells. Indeed, these cell types share most, if not all, the biochemical and regulatory features of the metabolic shift to fermentation: enhanced expression of glucose transporters and glycolytic enzymes, down-regulation of respiratory metabolism, and over-expression of the enzymes metabolizing pyruvate in the cytoplasm (either Pdc or Ldh) [Citation9,Citation10,Citation60].
5. Current ideas and their controversies
A rather speculative explanation for the occurrence of the Crabtree effect in yeast is the ecological perspective of the Make-Accumulation-Consume (MAC) hypothesis (a warfare strategy, according to the definition by [Citation14]). This assumes that some individual cells may exploit the ethanol toxicity to defend sugar rich resources from competitors, so justifying the sacrifice of some energy for this apparent advantage. This hypothesis derives from the observation that the whole genome duplication (WGD) event in yeast evolution coincides with the diversification of angiosperms, so that post-WGD lineages of yeasts have a significant Crabtree effect [Citation26,Citation61], and from studies on the kinetics properties of ancestral Adh [Citation62]. Main arguments against MAC hypothesis are that ethanol is produced also at low, non-toxic concentrations, many other examples of metabolic shifts that involve end-products not as toxic as ethanol exist, and, above all, the observation that in monoculture laboratory populations, where there is no need for competition, aerobic fermentation is fully maintained [Citation14].
A similar ecological perspective has also been evoked as a possible explanation for the Warburg effect in relation to the tumour microenvironment, since acidosis due to lactate production may alter the surrounding healthy cells, favouring the tumour cells [Citation16]. It is interesting that the main argument against this view of Warburg effect was referred to yeast monocultures (conditions completely isolated from the environment) in glucose-limited chemostats at high dilution rates, where aerobic fermentation is not lost [Citation16].
Not only the useful impact of by-products of the inefficient metabolism, but also the negative impact of by-products of respiration, the reactive-oxygen-species (ROS), has been considered to explain the metabolic shift to aerobic fermentation, though this idea does not explain why cells still respire during growth [Citation12]. The negative impact of ROS leading to aerobic glycolysis has been particularly explored in the case of tumour cells [Citation63] and also in yeast [Citation64] demonstrated that in colonies of S. cerevisiae, repression of respiration and ROS-scavenging via glutathione inhibited apoptosis and conferred a survival advantage.
The other notable view on the Crabtree effect in yeast is the so-called rate/yield trade off hypothesis (RYT) [Citation65]. Starting from the assumptions that growth rate maximization is a selective advantage for the population and growth rate is proportional to the ATP production rate, RYT is based on fundamental thermodynamic constraints of ATP production, which show that maximal ATP production rates are attained at intermediate yields of ATP [Citation66]. Consequently, maximal growth rate is achieved at intermediate ATP yields, so giving reason for the existence of energy-spilling metabolic strategies, such as aerobic fermentation. The question is to account for the maintenance of an energy-efficient metabolism (the exact contrary of the MAC hypothesis). So, the authors hypothesize that the energy-efficient metabolism is active in structured environments such as solid substrates, where selection for yield instead of growth rate may constitute a selective advantage. The coexistence of energy-efficient and inefficient cells was shown by MacLean and Gudelj [Citation67] in their competition experiments with different yeast mutants, and has been considered as a strong support for RYT [Citation15].
However, the main weakness for RYT (as for MAC), is represented by the metabolic strategies adopted by monocultures. In fact, contrarily to RYT previsions, the prolonged glucose-limited chemostat cultures at low dilution rates still maintain the energy-efficient metabolism of respiration [Citation68].
The idea that growth rate maximization is the main objective of the population also underlies the “economical approach” by Molenaar et al. [Citation14], which is based on the concept of resource allocation to optimize growth. The metabolic shift is viewed as the result of a trade-off between energetic efficiency of a pathway and the costs invested in synthesizing enzymes for the pathway. Based on these assumptions, they develop a model, which takes into account that efficient pathways need more cellular machinery to operate (e.g. the mitochondrion in yeast), so that, at low substrate concentrations, efficient metabolism leads to lower growth rate, and at high substrate concentrations inefficient metabolism leads to a higher growth rate. Following a similar approach, based on the hypothesis that cells are constrained by the allocation of protein mass, recently Nilsson and Nielsen [Citation69] have proposed a stoichiometric model that quantitatively predicts the Crabtree effect. Moreover, according to this model, the protein content of the Crabtree-negative yeast Kluyveromyces marxianus (up to 72% of cell mass, compared with only about 46% in S. cerevisiae) [Citation70] could explain the absence of the Crabtree effect in this yeast, since the Crabtree effect is only predicted to occur if proteins are limiting.
Notwithstanding the “economical approach” is able to predict the metabolic shifts in several cases, it remains to demonstrate if metabolic costs of different pathways are indeed so different. The same authors report the case of Lactococcus lactis, where protein costs do not explain the shift from mixed acid to homo-lactic fermentation [Citation14].
Another proposal, taken in great consideration especially in the case of the Warburg effect, is that the latter would represent an adaptation mechanism to support the biosynthetic requirements of fast proliferation. According to this view, the high glycolytic flux associated to fermentation conveys large amounts of glucose to the anabolic processes needed to sustain rapid population growth [Citation8]. In particular, the branching biosynthetic patterns from glycolysis intermediates of PPP (Pentose Phosphate Pathway), hexosamine and serine synthesis pathways have been reported in this context [Citation71]. The concept of an efficient allocation of resources for biosynthesis has been extended to the case of metabolic shift in E. coli with support by quantitative proteomic analyses [Citation72,Citation73]. However, some recent studies demonstrate that the cost of protein production for aerobic glycolysis is huge, whereas biosynthetic programs in cell require lower amount of proteins [Citation74]. Consequently, the major limitation of the interpretation of Warburg effect as optimization of biosynthesis resides in the fact that most of the carbon is indeed excreted in the form of lactate as end-product and not retained [Citation75]. Moreover, it is also widely accepted that mitochondria play a role for the biosynthetic program of proliferating cells with Krebs cycle acting as another hub for biosynthesis [Citation40]. In light of these considerations, the idea that the Warburg effect can be only explained by the necessity to feed the branching pathways of biosynthesis does not seem fully justified.
It is worth noting that the above-mentioned ideas, proposed to explain the Crabtree/Warburg effect, often base their attractiveness on the apparent correlation between aerobic glycolysis and cell proliferation together with accelerated biosynthetic pathways. However, the phenomenological observation that fast proliferating cells are fermentative usually derives from the incorrect idea that fermentation per se is equal to high proliferation rates (as well as high biosynthetic rates). Indeed, according to the Monod model [Citation76], the proliferation rate of a cell population is the direct consequence of the availability of nutrients. So, when the glucose uptake rate is high, a high growth rate is observed corresponding to higher biosynthetic rates as well. In addition, since the physical constraint represented by the respiration “bottleneck” exists (B), the excess glucose (overflow) is unavoidably channelled along the fermentation pathway. In conclusion, the occurrence of the fermentative metabolism at high growth rates is not the cause, but merely a consequence of the metabolic shift under high nutrient conditions, as also indicated by the weak correlation found between ethanol production rate and growth rate in the case of yeasts [Citation77] ().
Figure 3. Ethanol production rate vs. growth rate of several strains of S. cerevisiae in different environmental conditions (Data from [Citation77]).
![Figure 3. Ethanol production rate vs. growth rate of several strains of S. cerevisiae in different environmental conditions (Data from [Citation77]).](/cms/asset/f0962867-a9e7-4d2d-b717-8d79618beb5f/kccy_a_1442622_f0003_b.gif)
So, if the short-term effect is rather easy to explain, basically due to the physical constraint represented by the mitochondrial processing capacity, it remains to elucidate the fitness advantage of the active mechanisms that repress, in the long-term, the energy-efficient respiration.
6. Sugar-induced cell death: history and proposed mechanisms
Yeast cells enter a stationary phase (G0) when essential nutrients are depleted [Citation78,Citation79], but resume a new proliferative phase when nutrient conditions turn favourable. Aiming at identifying the nutrients and signals determining the new proliferative phase, in 1991, Granot and Snyder [Citation18] described the singular phenomenon of a rapid loss of cell viability, when the stationary cells were incubated in the presence of glucose only, in the absence of additional nutrients to support growth. Instead, the same cells, when transferred to water, remained viable for weeks (). The phenomenon was termed sugar-induced cell death (SICD), and observed in several yeast strains, in spores, at temperatures ranging from 25 to 37 °C, and also in the presence of other carbon sources [Citation18,Citation19].
Figure 4. S. cerevisiae cell viability under different conditions: cells incubated in pure water (open circles); in water and glucose (closed circles); in water, glucose and phosphate (open triangles). Data from [Citation18] and [Citation21].
![Figure 4. S. cerevisiae cell viability under different conditions: cells incubated in pure water (open circles); in water and glucose (closed circles); in water, glucose and phosphate (open triangles). Data from [Citation18] and [Citation21].](/cms/asset/f66d5d98-8a38-41b6-bec6-37221a21601d/kccy_a_1442622_f0004_b.gif)
SICD was confirmed in another paper by Granot et al. [Citation20], who showed that glucose induced rapid apoptosis in stationary yeast cells, the latter characterized by production of ROS and other typical apoptotic markers. The authors claimed that ROS were not sufficient for SICD commitment, since yeast cells were still able to survive when transferred back to water. SICD was observed to occur also in the case of the bottom fermenting yeast Saccharomyces pastorianus, not only in aerobic but in anaerobic conditions as well [Citation80].
More recently, Lee et al. [Citation21] using the same experimental conditions to induce SICD in stationary yeast cells (37 °C, aerobiosis), measured a higher O2 consumption rate and ROS production in glucose/water incubated cells than in stationary cells in water (0.86 vs. 0.26 mmol O2 g−1 cells h−1). According to the authors, the precocious death of glucose/water cells was because glucose, in the absence of other nutrients, failed to down-regulate respiration (this explaining the high O2 consumption and consequent ROS production) in contrast to what generally observed when glucose exerts both short and long-term Crabtree effect. Further, the same authors showed that addition of phosphate to the cells in glucose/water significantly delayed SICD (). With addition of phosphate, it was observed an increase in the synthesis of fructose-1,6-diphosphate and a decrease both in O2 consumption (down to a value of 0.24 mmol O2 g−1 cells h−1) and ROS accumulation. According to the authors, this evidenced that fructose-1,6-diphosphate could down-regulate respiration ad so delay SICD.
Further, in 2012 Santos and co-authors [Citation81] described a phenomenon of loss of viability similar to SICD but induced by NH4+ upon transfer of aged Saccharomyces cerevisiae cells in water containing only ammonium sulphate. The toxic effect of NH4+ was positively correlated with its concentration and particularly significant for cells starved for auxotrophic-complementing amino acids. The data indicated that NH4+ in water inhibited induction of autophagy, but this inhibition was not the cause of cell death. The authors claimed that cell death was initially apoptotic, but followed by extensive necrosis, the latter accompanied by ATP depletion. About the mechanism involved in ammonium-induced-cell-death, the authors provide results indicating that in amino acids starved cells, NH4+ activates PKA and TOR signalling cascades.
In summary, the above mentioned literature state: i) the attribution of SICD to ROS production, due to the enhanced O2 consumption [Citation21]; ii) the occurrence of SICD also in anaerobic conditions [Citation80] (a strong argument against the role of ROS produced during respiration as the only responsible for SICD); iii) the protective role of phosphate towards SICD [Citation21]; iv) the involvement of the nutrient signalling cascades (PKA/TOR) in the case of ammonium-induced-cell-death; v) the observation that cell death was predominantly necrotic and accompanied by ATP depletion [Citation81].
We believe that the different cases of SICD should be seen as outcomes of a common functional mechanism related to the integrated effects of the nutrient signalling cascades PKA/TOR on ATP balance. As known, cAMP/Ras/PKA pathway occupies a crucial position in the response to glucose and other essential nutrients such as ammonium [Citation82]. Moreover, the TOR pathway (TORC1) is thought to integrate the overall nutritional and energy status of the cell [Citation83,Citation84]. Therefore, borrowing the evidences by Santos et al. [Citation81] on ammonium-induced-cell-death, we suggest that also sugar-induced cell death may be mainly due to ATP depletion, this explaining the case of SICD occurring in anaerobiosis [Citation80].
ATP depletion is a common mechanism leading to cell death in several scenarios [Citation85–87]. Cell death due to ATP depletion can occur through necrosis or apoptosis [Citation88], and is mainly related to calcium homeostasis [Citation89–91]. The control of the low levels of intracellular calcium is mainly exerted by plasma membrane Ca2+-ATPase (PMCA), in an ATP dependent manner [Citation90]. Upon ATP depletion, Ca2+ will accumulate in the mitochondria and activate cell death [Citation90,Citation91].
depicts our integrated view of the processes involved in the death of yeast cells under the three conditions reported in water (no nutrients), glucose only, glucose and phosphate, respectively.
Figure 5. Schematic simplified representation of S. cerevisiae metabolic activity in the different conditions of (A) water; B) glucose; C) glucose + NaH2PO4) and corresponding days of survival until complete loss of viability. Glu, glucose; G6P, glucose 6-phosphate; F6P, fructose 6-phosphate; F1,6BP, fructose 1,6-biphosphate; GA3P, glyceraldehyde 3-phosphate; DHAP, dihydroxyacetone phosphate; Pyr, pyruvate; Eth, ethanol; Ac-CoA, acetyl-coenzyme A; ROS, reactive oxygen species.
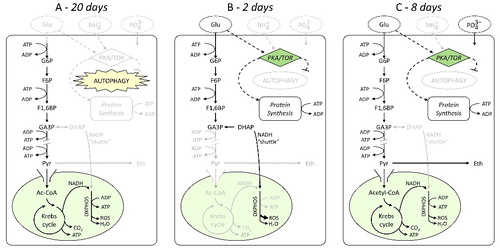
Water (no nutrients) (A) – Stationary-phase yeast cells incubated in water at 37 °C show a slow, but progressive loss of viability that leads to complete death of the population after approximately 20 days (, open circles). The complete lack of nutrients inhibits the activation of the PKA/TOR nutrient signalling cascade (for a complete review of nutrient sensing and signalling see [Citation92,Citation93]) which, on the one hand, reduces all the metabolic processes to the minimum required for cell survival and, on the other hand, induces the activation of the autophagy pathway which starts the recycling of reserve compounds to satisfy the cell requirements and maintain active respiration (O2 consumption rate after 6 h of incubation is 0.26 mmol g−1 h−1). Since the available resources are limited, the cell population slowly loses viability until autophagy is able to supply resources for the basic maintenance requirements.
Glucose only (B) – Cells incubated in a solution at high glucose concentration show a sudden loss of viability leading to complete death after only 2 days (, closed circles). In this case, the presence of glucose activates the PKA/TOR nutrient signalling cascade. This event results in the activation/acceleration of several anabolic and catabolic pathways and in the inhibition of autophagy. Such conditions induce a very rapid depletion of the available energy resources (ATP) which cannot be replenished due to lack of phosphate. Moreover, at high glucose concentration, a high rate of oxygen consumption (0.84 mmol g−1 h−1 after 6 h of incubation) and production of ROS are observed [Citation21]. Since the glycolytic reactions downstream GA3P are not possible due to the lack of phosphate, the higher O2 consumption can be explained only by the glycerol-3-phosphate (Gly-3-P)/dihydroxyacetone phosphate (DHAP) shuttle which provides NADH produced in the cytosol to the mitochondrial electron transport chain [Citation94].
Glucose and phosphate (C) – The addition of phosphate to the glucose solution effectively increases the survival of the yeast culture, showing complete death of the population after 8 days (, open triangles). Differently from the previous case, the presence of inorganic phosphate in the medium allows the cells to regenerate ATP. The GA3P downstream reactions are now possible, so that pyruvate can be respired: oxygen consumption is the same as in the case A after 6 h of incubation [Citation21]. Due to the presence of the “bottleneck”, the pyruvate overflow is also channelled along the fermentation pathway. Fructose-1,6-biphoshate (F1,6BP) concentration results three-fold higher than in case A, so it is likely that it may induce down-regulation of respiration in the long term [Citation54]. However, the concomitant activation of the PKA/TOR nutrient signalling cascade by glucose accelerates the metabolic flux leading to ATP consumption similar to case B, but contrarily to this latter, ROS production is not enhanced. The result is in an increased viability than with glucose alone.
Overall, the analysis of all these experiments shows that cell death can be ascribed to the same causal mechanism, with different timings related to different rates of ATP exhaustion. Differently from Lee et al. [Citation21], who ascribes the pro-survival function of the Crabtree effect to the down-regulation of respiration, and consequently to a lower production of ROS, we propose that the reduction of ROS may only be a side-advantage.
In this context, it is interesting that caloric restriction (growth in the presence of low glucose) seems not to affect the longevity of the Crabtree-negative yeast Kluyveromyces lactis, but significantly increases that of the Crabtree-positive S. cerevisiae [Citation95]. Indeed, SICD in Crabtree-negative yeasts, which are able to control glucose uptake [Citation25,Citation26], results significantly delayed if compared to S. cerevisiae (2016 personal observation by E de Alteriis, unreferenced).
We suggest that the rationale behind our SICD interpretation can be relevant also to explain the Crabtree/Warburg effect.
7. Glucose flux dynamics
Both yeast and mammalian cells displaying aerobic fermentation are characterized by high glycolytic fluxes, which in turn are generated by high glucose uptake rates [Citation96,Citation97]. In these conditions, both cell types exhibit an enhanced expression of glucose transporters. For example, in some tumour cells, overexpression of different glucose transporters have been reported [Citation98–102]. Regarding the glycolytic flux modulation, the main enzymes controlling the three irreversible reactions in the glycolytic sequence, namely hexokinase (HK), phosphofructokinase (PFK) and pyruvate kinase (PK), have been shown to be overexpressed in a number of tumour cells as well as in fermenting yeast [Citation10]. Indeed, there are some controversies on the rate of the last committed step of glycolysis, catalysed by PK, which in some tumour cells results to be attenuated (due to the occurrence of the isoform PKM2) despite the overall increased glycolysis rate [Citation71]. However, such contradiction is solved by taking into account other alternative reactions converting PEP into pyruvate [Citation103] thus compensating the above-mentioned attenuation [Citation9].
In the case of yeast, it is known that glycolytic flux is readily increased when yeast cells are exposed to excess of glucose both in chemostat and in batch culture [Citation104]. The increased glycolytic flux in response to glucose excess shows many metabolic similarities with the increase following oxygen depletion [Citation105].
The correlation between Crabtree effect and glucose-uptake rate has been strictly elucidated in quantitative terms by Huberts et al. [Citation77] in yeasts, analysing data from an extensive number of cases reported in the literature. They found a very significant correlation between ethanol production rate and sugar uptake rate (). Noteworthy, the correlation between fermentation and glucose uptake rates holds independently of culturing methods (chemostat, batch), sugar concentration in the medium, type of sugar (glucose, maltose, galactose, sucrose), strain used, environmental conditions (pH, temperature, aerobiosis, anaerobiosis), and it is also maintained in the case of both Crabtree-positive and negative species. This suggests that Crabtree-negative yeasts have simply a lower sugar import rate, in line with what was suggested earlier [Citation25,Citation106].
Figure 6. (A) Ethanol production rate vs. sugar uptake rate in different S. cerevisiae cultures (dataset from [Citation77]); (B) Lactate production vs. sugar uptake rate in in vitro preparations of rat small intestine (data from [Citation107]).
![Figure 6. (A) Ethanol production rate vs. sugar uptake rate in different S. cerevisiae cultures (dataset from [Citation77]); (B) Lactate production vs. sugar uptake rate in in vitro preparations of rat small intestine (data from [Citation107]).](/cms/asset/6b7aacff-3ae2-4c30-a58f-2516dea33630/kccy_a_1442622_f0006_b.gif)
In the case of mammalian cells, some evidence of a similar correlation between glucose uptake and fermentation rates is also present in the literature, as shown by Hanson and Parsons [Citation107] during their experiments on in vitro preparations of rat small intestine (B). As far as we know, no clear studies on this particular subject have been performed with proliferative cancer cell. However, Wang et al. [Citation108] in their study on colorectal cancer cell cultures, reported a correlation between glucose consumption and LdhA activity, which can be assumed as a proxy of the lactate production rate. Furthermore, it is well known that in mammalian cell culture technology, the glucose-limited fed-batch technique can be used to significantly reduce the formation of lactate [Citation109].
Coming back to yeasts, already in 1992, Fiechter and Seghezzi [Citation25], discussing the different behaviour of Crabtree-positive and negative yeasts, emphasized the necessity to think in terms of glucose flux dynamics rather than static concentrations, pointing out the relevance of glucose uptake rate, in turn determining the “overflow” metabolism. Recently, Hagman and Piskur [Citation26], in their evolutionary study on the appearance of Crabtree effect in yeast species, grouped them in either Crabtree-positive (respiro-fermenting) or -negative (purely respiring) based on a cut-off value for glucose uptake rate.
Besides the apparent relevance of sugar uptake in modulating the fermentation process, Huberts et al. [Citation77] also underline the importance of the glycolytic flux, driven by sugar uptake rate, in determining the Crabtree effect. They report that both the glycolytic flux between glucose-6-phosphate (G6P) and fructose-6-phosphate (F6P) and the concentration of fructose-1,6-biphoshate (F1,6BP) are strongly correlated with the sugar uptake rate, identifying F1,6BP as the glycolytic intermediate able to regulate the metabolic shift between respiration and fermentation. In fact, a glucose pulse to a glucose-limited chemostat induces an increased influx rate, higher F1,6BP concentration, and concomitant onset of ethanol production [Citation110,Citation111]. Furthermore, very recently, comparative study of yeast and cancer cells pointed out how high levels of F1,6BP couple the glycolytic flux to the activation of Ras regulating cell proliferation [Citation112].
In general, in yeast, in conditions of high glycolytic fluxes, the upper glycolytic intermediates (G6P, F6P, F1,6BP) and pyruvate increase, whereas the lower metabolites, 2-phoshoglycerate (2PG), 3-poshoglycerate (3PG), and phosphoenolpyruvate are relatively less abundant [Citation105]. However, it is interesting to point out that, in contrast to the observed increases of glucose fluxes and related FBP levels, the concentrations of ATP, ADP and AMP do not show any clear trend related with the sugar uptake rate. Also, the redox balance NAD/NADH has a limited role in the establishing the Crabtree effect, since changes in NAD+ has only minor effects on the correlation between glycolytic flux and ethanol production rate [Citation77,Citation113–115].
8. A dynamic perspective of the Crabtree/Warburg effect
To summarize all the topics reported above, on the one hand we evidenced how the Crabtree/Warburg effect is strongly associated to an enhanced glucose uptake rate and consequent high glycolytic flux; on the other hand, we described the phenomenon of SICD as consistently caused by ATP crisis. In our opinion, the two seemingly unrelated phenomena of Crabtree/Warburg effect and SICD, when considered together, are the key to provide a plausible explanation for the fitness advantage of the former effect, thus resolving the apparent paradox of the active repression of the energy-efficient respiration in favour of the energy-inefficient fermentation. This becomes clear if the effect, rather than in static conditions, is analysed within a dynamic representation of the main metabolic fluxes with the related ATP balance per unit of time at either low or high levels of glucose uptake ().
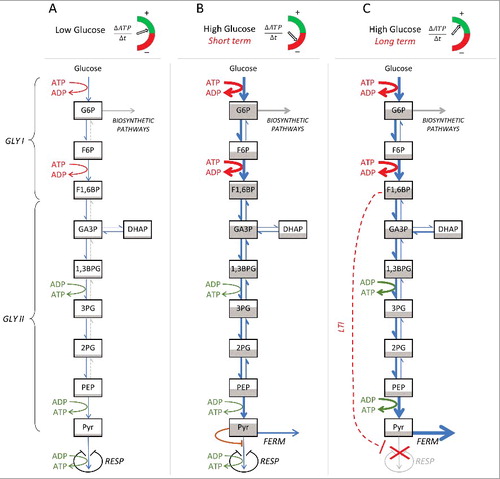
Figure 7. Conceptual representation of glycolysis and associated ATP production balance according to glucose availability. (A) At low glucose uptake rate, the metabolism is fully respiratory, and the ATP balance is positive. (B) Following an increase in glucose availability and consequent higher glucose uptake rate, in the short term, the irreversible reactions of the first phase of glycolysis (GLY I) induce a higher rate of ATP consumption, compared to the ATP production rates during the second phase of glycolysis (GLY II); pyruvate is respired, but its surplus (“overflow”) is fermented. The brown line represents the reduced affinity of the enzyme pyruvate dehydrogenase for high concentrations of pyruvate (“bottleneck”). (C) In the long term at high glucose concentration, the respiration is down-regulated (red dashed line, long term inhibition, LTI) by the accumulation of F1,6BP. Concomitantly, fermentation is increased allowing for a faster conversion of pyruvate which, in turn, increases the flows of the second phase of the glycolysis (GLY II), thus leading to the restoration of a positive balance of ATP production. RESP, respiration; FERM, fermentation; G6P, glucose 6-phosphate; F6P, fructose 6-phosphate; F1,6BP, fructose 1,6-biphosphate; GA3P, glyceraldehyde 3-phosphate; DHAP, dihydroxyacetone phosphate; 1,3BPG, 1,3-bisphosphoglycerate; 3PG, 3-phosphoglycerate; 2PG, 2-phosphoglycerate; PEP, phosphoenolpyruvate; Pyr, pyruvate.
The usefulness to describe dynamically the metabolic network of a cell has been clearly recognized by the early work on Metabolic Flux Analysis [Citation116] and by the so-called “fluxomic” that, within the more general context of Systems Biology, specifically refers to the study of rates of metabolic fluxes [Citation117]. This approach provides a dynamic view of otherwise traditionally static metabolomic data [Citation118], being based on the balance of fluxes influencing intracellular metabolites stoichiometrically determined by different advanced techniques (e.g. steady/non-steady state analysis, isotopic flux balance using 13C-labeled tracers) [Citation119].
In full agreement with the necessity of a dynamic perspective in the study of cell metabolism, here we propose a description of glycolysis according to the approach of System dynamics (sensu Forrester [Citation120]), i.e. a model based on stocks/compartments representing the value (or state) of the considered variables (i.e. metabolites) over time and flows/arrows representing the rates of increase or decrease of the same variables, including the feedback loops between the different components of the system. In particular, this approach is useful to emphasize the nonlinear behaviour of complex systems due to feedback effects following changing input levels. For example, SD models were found to be useful to represent transient metabolism dynamics in both plants [Citation121] and yeast [Citation58].
In we focus on the key reactions and metabolites upstream of pyruvate, the latter being the central metabolic hub at the crossroads between respiration and fermentation. It is evident how different reactions rates (i.e. flows) result in changing levels of intermediate compounds. In other words, the balance between in- and out-fluxes around each metabolite defines its accumulation/depletion, thus influencing the upstream and downstream reactions by feedbacks like, for instance, substrate induction or product repression of reactions.
In this context, the critical point is represented by the different nature of the reactions that compose the glycolytic pathway. During the first phase of glycolysis (GLY I), the reactions catalysed by hexokinase and phosphofructokinase which lead to glucose-6P (G6P) and fructose-1,6-BP (F1,6BP), respectively, are irreversible, whereas the reactions of the second phase of glycolysis (GLY II) that lead to pyruvate are reversible, with the exception of the last one catalysed by pyruvate kinase. It is relevant that the reversible reactions of the pathway are very sensitive to changes in the concentrations of the pathway intermediates [Citation23]. This different reaction kinetics determine continuous adjustments of flow directions and rates. On the contrary, the two irreversible reactions of GLY I, being not down-regulated by product concentration levels, produce unavoidable accumulation of F1,6BP, when the glucose uptake rate happens to be greater than the outflow from the F1,6BP stock to the downstream GLY II pathway.
Starting from a condition of low glucose uptake and consequent generally low glycolytic flux (A), the mitochondrial machinery is able to process all produced pyruvate without any accumulation and/or overflow setting. Under such conditions, cells can be considered in a sustainable energetic balance because the ATP production occurs at low rate, but high efficiency (the metabolism is fully respiratory). The rate of ATP production ΔATP/Δt) in this case is positive and capable to maintain the costs of the biosynthetic pathways, mostly branching from G6P (A).
Higher glucose concentrations determine higher glycolytic flux which overtakes the mitochondrial processing capability (“bottleneck”), thus producing pyruvate accumulation. In the short-term, this condition produces the co-occurrence (“overflow”) of respiration and fermentation (B, see also B). Under such conditions, the energetic balance becomes negative, because of the unbalanced rate of GLY I and GLY II. In fact, the high glucose uptake rate accelerates the reactions catalysed by hexokinase and phosphofructokinase during GLY I, thus proportionally increasing the rate of ATP consumption. On the other hand, the ATP production of GLY II is limited by the lowering rates of the reversible reactions due to the accumulation of pyruvate and, consequently, of the upstream intermediates. Also, a higher energetic cost under such conditions is due to the increased flux to the biosynthetic pathways branching from G6P (B). Moreover, the ATP production capacity of the mitochondria is saturated.
This condition is clearly unsustainable in the long-term because it would induce SICD by ATP depletion. Then, the reasons for the onset of “long-term inhibition of respiration” become evident as a restoration of ATP balance. This is possible by the down-regulation of respiration allowing an increased allocation of cell resources to fermentation (C). In fact, in this way, the rate of pyruvate removal can be increased permitting higher fluxes in GLY II, with corresponding increased ATP production rates which compensate the higher consumption in GLY I at high glucose concentration.
In full agreement with this view, following exposure to excess glucose and increased glycolytic flux, an initial drop in the ATP level has been reported [Citation122]. Later, the concentration of ATP is restored due to an increase in the specific ATP production rate (qATP) [Citation123]. This suggests the occurrence of feedback mechanisms to keep constant the ATP level in the cell [Citation77]. As mentioned in a previous section, Curi et al [Citation59] reported in proliferative mammalian cells an increase of the glycolytic flux greatly surpassing the Vmax of Pdh and commented that the consequent production of lactate could be a way to avoid accumulation of pyruvate. In our view, the accumulation of pyruvate is not harmful per se, but it triggers a chain reaction that leads to the build-up of the upstream glycolytic intermediates that slows down the ATP-producing reactions of GLY II.
In conclusion, the fitness advantage of aerobic fermentation under high glucose concentrations results evident if the ATP balance at high glycolytic fluxes is taken into account. Such logical reasoning on the system dynamics suggests that the Crabtree/Warburg effect may be interpreted as the avoidance of ATP depletion in those conditions where glucose uptake is higher than the downstream processing capability of the second phase of glycolysis. It follows that the down-regulation of respiration is strategic for cell survival allowing the allocation of more resources to the fermentation pathway, thus maintaining the cell energetic homeostasis.
9. Conclusions
Focusing on the dynamics of the glycolytic flux, this study shows how the Crabtree/Warburg effect may be interpreted as an escape strategy from death, the latter due to the ATP depletion determined by glucose excess.
More generally and once again, glucose reveals to play a central role in cell longevity, with the functional mechanism behind such role indeed mediated by the maintenance of ATP homeostasis.
A general tendency exists to shift from an energy-efficient pathway to an energy-inefficient one, since it has been observed that several microorganisms growing at high substrate concentration divert a considerable amount of glucose to incompletely oxidized end-products. In analogy to what discussed for the Crabtree/Warburg effect in yeast and mammalian cells, it is presumable that these metabolic shifts may represent the survival strategy selected for its fitness advantage in glucose rich environments.
Besides the Crabtree/Warburg effect, organisms may have evolved other strategies to avoid the ATP crisis due to unbalanced fluxes, for example the upstream regulation of glucose uptake observed in Crabtree-negative yeasts. Indeed, the regulation of glucose uptake seems to play a very significant role also in mammalian cells: in non-proliferative cells the low expression of glucose transmembrane transporters is observed, whilst in different cancer models, glucose transporters are overexpressed in the tumour cells, but downregulated in the healthy surrounding tissues. Such differential behaviour could provide key insights to devise treatment strategies to preferentially target cancer cells over healthy cells.
In conclusion, this interpretation of the Warburg effect reinforces the need to focus on glucose metabolism as a therapeutic target. Moreover, a thorough investigation of glycolytic fluxes and their dynamic modelling bears major interest to identify critical points for a metabolic therapy producing differential effects on healthy and tumour cells.
Disclosure of potential conflicts of interest
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Acknowledgements
EdA and FC contributed equally to the work. This research was partially supported the project MOD_DEV_CELL “System dynamics modeling of microbial cell cultures: numerical methods, process optimization, and individual-based approach” financed by University of Naples “Federico II”, 2017–2019.
References
- Warburg O, Negelein E. Bemerkung zu einem Aufsatz von F. Weigert Zeitschrift für Phys Chemie. 1924;108U:101–102.
- Warburg O. The metabolism of carcinoma cells. J Cancer Res. 1925;9:148–163. doi:10.1158/jcr.1925.148.
- Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi:10.1085/jgp.8.6.519. PMID:19872213
- Warburg O. On the Origin of Cancer Cells. Science. 1956;123:309–314. doi:10.1126/science.123.3191.309. PMID:13298683
- Crabtree HG. Observations on the carbohydrate metabolism of tumours. Biochem J. 1929;23:536–545. doi:10.1042/bj0230536. PMID:16744238
- De Deken RH. The Crabtree effect: a regulatory system in yeast. J Gen Microbiol. 1966;44:149–156. doi:10.1099/00221287-44-2-149. PMID:5969497
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, et al. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi:10.1016/j.cmet.2007.10.002. PMID:18177721
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi:10.1126/science.1160809. PMID:19460998
- Diaz-Ruiz R, Uribe-Carvajal S, Devin A, et al. Tumor cell energy metabolism and its common features with yeast metabolism. Biochim Biophys Acta – Rev Cancer. 2009;1796:252–265. doi:10.1016/j.bbcan.2009.07.003.
- Diaz-Ruiz R, Rigoulet M, Devin A. The Warburg and Crabtree effects: on the origin of cancer cell energy metabolism and of yeast glucose repression. Biochim Biophys Acta – Bioenerg. 2011;1807:568–576. doi:10.1016/j.bbabio.2010.08.010.
- Vander Heiden MG, DeBerardinis RJ. Understanding the intersections between metabolism and cancer biology. Cell. 2017;168:657–669. doi:10.1016/j.cell.2016.12.039. PMID:28187287
- Goel A, Wortel MT, Molenaar D, et al. Metabolic shifts: a fitness perspective for microbial cell factories. Biotechnol Lett. 2012;34:2147–2160. doi:10.1007/s10529-012-1038-9. PMID:22936303
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi:10.1016/j.cell.2011.02.013. PMID:21376230
- Molenaar D, van Berlo R, de Ridder D, et al. Shifts in growth strategies reflect tradeoffs in cellular economics. Mol Syst Biol. 2009;5:323. doi:10.1038/msb.2009.82. PMID:19888218
- Pfeiffer T, Morley A. An evolutionary perspective on the Crabtree effect. Front Mol Biosci. 2014;1:17. doi:10.3389/fmolb.2014.00017. PMID:25988158
- Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi:10.1016/j.tibs.2015.12.001. PMID:26778478
- Alberghina L, Mavelli G, Drovandi G, et al. Cell growth and cell cycle in Saccharomyces cerevisiae: basic regulatory design and protein-protein interaction network. Biotechnol Adv. 2012;30:52–72. doi:10.1016/j.biotechadv.2011.07.010. PMID:21821114
- Granot D, Snyder M. Glucose induces cAMP-independent growth-related changes in stationary-phase cells of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991;88:5724–5728. doi:10.1073/pnas.88.13.5724. PMID:1648229
- Granot D, Snyder M. Carbon source induces growth of stationary phase yeast cells, independent of carbon source metabolism. Yeast. 1993;9:465–479. doi:10.1002/yea.320090503. PMID:8322510
- Granot D, Levine A, Dorhefetz E. Sugar-induced apoptosis in yeast cells. FEMS Yeast Res. 2003;4:7–13. doi:10.1016/S1567-1356(03)00154-5. PMID:14554192
- Lee YJ, Burlet E, Galiano F, et al. Phosphate and succinate use different mechanisms to inhibit sugar-induced cell death in yeast: insight into the Crabtree effect. J Biol Chem. 2011;286:20267–20274. doi:10.1074/jbc.M110.209379. PMID:21515692
- Verduyn C, Stouthamer AH, Scheffers WA, et al. A theoretical evaluation of growth yields of yeasts. Antonie Van Leeuwenhoek. 1991;59:49–63. doi:10.1007/BF00582119. PMID:2059011
- Voet D, Voet JG. Biochemistry. New York (NY): Wiley; 1990.
- van Dijken J, Bauer J, Brambilla L, et al. An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enzyme Microb Technol. 2000;26:706–714. doi:10.1016/S0141-0229(00)00162-9. PMID:10862876
- Fiechter A, Seghezzi W. Regulation of glucose metabolism in growing yeast cells. J Biotechnol. 1992;27:27–45. doi:10.1016/0168-1656(92)90028-8.
- Hagman A, Piškur J. A study on the fundamental mechanism and the evolutionary driving forces behind aerobic fermentation in yeast. PLoS One. 2015;10(1):e0116942. doi:10.1371/journal.pone.0116942.
- Compagno C, Dashko S, Piškur J. Introduction to carbon metabolism in yeast. In: Pišku J, Compagno C, editors. Molecular mechanisms in yeast carbon metabolism. Berlin: Heidelberg: Springer; 2014. p. 1–19.
- Silva LS, Goncalves LG, Silva F, et al. STAT3:FOXM1 and MCT1 drive uterine cervix carcinoma fitness to a lactate-rich microenvironment. Tumor Biol. 2016;37:5385–5395. doi:10.1007/s13277-015-4385-z.
- Johnson JM, Cotzia P, Fratamico R, et al. MCT1 in invasive ductal carcinoma: monocarboxylate metabolism and aggressive breast cancer. Front Cell Dev Biol. 2017;5:27. doi:10.3389/fcell.2017.00027. PMID:28421181
- Lopes-Coelho F, Nunes C, Gouveia-Fernandes S, et al. Monocarboxylate transporter 1 (MCT1), a tool to stratify acute myeloid leukemia (AML) patients and a vehicle to kill cancer cells. Oncotarget. 2017;8:82803–82823. doi:10.18632/oncotarget.20294. PMID:29137304
- Pinheiro C, Longatto-Filho A, Azevedo-Silva J, et al. Role of monocarboxylate transporters in human cancers: state of the art. J Bioenerg Biomembr. 2012;44:127–139. doi:10.1007/s10863-012-9428-1. PMID:22407107
- Rogatzki MJ, Ferguson BS, Goodwin ML, et al. Lactate is always the end product of glycolysis. Front Neurosci. 2015;9:1–7. doi:10.3389/fnins.2015.00022. PMID:25653585
- Porporato PE, Dhup S, Dadhich RK, et al. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front Pharmacol. 2011;2:49. doi:10.3389/fphar.2011.00049. PMID:21904528
- Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, et al. Energy metabolism in tumor cells. FEBS J. 2007; 274(6):1393–1418. doi:10.1111/j.1742-4658.2007.05686.x. PMID:17302740
- Yu M, Shi Y, Wei X, et al. Depletion of mitochondrial DNA by ethidium bromide treatment inhibits the proliferation and tumorigenesis of T47D human breast cancer cells. Toxicol Lett. 2007;170:83–93. doi:10.1016/j.toxlet.2007.02.013. PMID:17391873
- Fogal V, Richardson AD, Karmali PP, et al. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol Cell Biol. 2010;30:1303–1318. doi:10.1128/MCB.01101-09. PMID:20100866
- Hoy AJ, Balaban S, Saunders DN. Adipocyte–tumor cell metabolic crosstalk in breast cancer. Trends Mol Med. 2017;23:381–392. doi:10.1016/j.molmed.2017.02.009. PMID:28330687
- Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi:10.1038/nrc.2016.73. PMID:27550820
- Lopes-Coelho F, Gouveia-Fernandes S, Serpa J. Metabolic cooperation between cancer and non-cancerous stromal cells is pivotal in cancer progression. Tumor Biol. 2018; Forthcoming. doi:10.1177/1010428318756203.
- DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2:e1600200. doi:10.1126/sciadv.1600200. PMID:27386546
- Sonveaux P, Végran F, Schroeder T, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. PMID:19033663
- Lee M, Yoon J-H. Metabolic interplay between glycolysis and mitochondrial oxidation: The reverse Warburg effect and its therapeutic implication. World J Biol Chem. 2015;6:148–161. doi:10.4331/wjbc.v6.i3.148. PMID:26322173
- Rabinowitz JD, Coller HA. Partners in the Warburg effect. Elife. 2016;5:e15938. doi:10.7554/eLife.15938. PMID:27073176
- Bononi A, Yang H, Giorgi C, et al. Germline BAP1 mutations induce a Warburg effect. Cell Death Differ. 2017;24:1694–1704. doi:10.1038/cdd.2017.95. PMID:28665402
- Amelio I. Genes versus environment: cytoplasmic BAP1 determines the toxic response to environmental stressors in mesothelioma. Cell Death Dis. 2017;e2907. doi:10.1038/cddis.2017.293. PMID:28661472
- Coller HA. Is cancer a metabolic disease? Am J Pathol. 2014;184:4–17. doi:10.1016/j.ajpath.2013.07.035. PMID:24139946
- Lemons JMS, Feng XJ, Bennett BD, et al. Quiescent fibroblasts exhibit high metabolic activity. Goodell MA, editor. PLoS Biol. 2010;8:e1000514. doi:10.1371/journal.pbio.1000514. PMID:21049082
- Simsek T, Kocabas F, Zheng J, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi:10.1016/j.stem.2010.07.011. PMID:20804973
- Pronk JT, Steensma HY, Van Dijken JP. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast. 1996;12:1607–1633. doi:10.1002/(SICI)1097-0061(199612)12:16%3c1607::AID-YEA70%3e3.0.CO;2-4. PMID:9123965
- Petrik M, Kappeli O, Fiechter A. An expanded concept for the glucose effect in the Yeast Saccharomyces uvarum: involvement of short- and long-term regulation. Microbiology. 1983;129:43–49. doi:10.1099/00221287-129-1-43.
- Postma E, Verduyn C, Scheffers WA, et al. Enzymic analysis of the crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol. 1989;55:468–477. PMID:2566299
- Santangelo GM. Glucose signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev MMBR. 2006;70:253–282. doi:10.1128/MMBR.70.1.253-282.2006. PMID:16524925
- Westergaard SL, Oliveira AP, Bro C, et al. A systems biology approach to study glucose repression in the yeast Saccharomyces cerevisiae. Biotechnol Bioeng. 2007;96:134–145. doi:10.1002/bit.21135. PMID:16878332
- Díaz-Ruiz R, Avéret N, Araiza D, et al. Mitochondrial oxidative phosphorylation is regulated by fructose 1,6-bisphosphate: a possible role in crabtree effect induction? J Biol Chem. 2008;283:26948–26955. doi:10.1074/jbc.M800408200. PMID:18682403
- Barford JP, Hall RJ. A mathematical model for the aerobic growth of Saccharomyces cerevisiae with a saturated respiratory capacity. Biotechnol Bioeng. 1981;23:1735–1762. doi:10.1002/bit.260230806.
- Sonnleitner B, Käppeli O. Growth of Saccharomyces cerevisiae is controlled by its limited respiratory capacity: Formulation and verification of a hypothesis. Biotechnol Bioeng. 1986;28:927–937. doi:10.1002/bit.260280620. PMID:18555411
- Pham HTB, Larsson G, Enfors SO. Growth and energy metabolism in aerobic fed-batch cultures of Saccharomyces cerevisiae: simulation and model verification. Biotechnol Bioeng. 1998;60:474–482. doi:10.1002/(SICI)1097-0290(19981120)60:4%3c474::AID-BIT9%3e3.0.CO;2-J. PMID:10099453
- Mazzoleni S, Landi C, Cartenì F, et al. A novel process-based model of microbial growth: self-inhibition in Saccharomyces cerevisiae aerobic fed-batch cultures. Microb Cell Fact. 2015;14:109. doi:10.1186/s12934-015-0295-4. PMID:26223307
- Curi R, Newsholme P, Newsholme EA. Metabolism of pyruvate by isolated rat mesenteric lymphocytes, lymphocyte mitochondria and isolated mouse macrophages. Biochem J. 1988;250:383–388. doi:10.1042/bj2500383. PMID:3128282
- Legiša M. Similarities and differences between cancer and yeast carbohydrate metabolism. In: Pišku J, Compagno C, editors. Molecular mechanisms in yeast carbon metabolism. Berlin, Heidelberg: Springer; 2014. p. 121–140.
- Piškur J, Rozpedowska E, Polakova S. et al. How did Saccharomyces evolve to become a good brewer? Trends Genet. 2006;22:183–186. doi:10.1016/j.tig.2006.02.002. PMID:16499989
- Thomson JM, Gaucher EA, Burgan MF, et al. Resurrecting ancestral alcohol dehydrogenases from yeast. Nat Genet. 2005;37:630–635. doi:10.1038/ng1553. PMID:15864308
- Gogvadze V, Zhivotovsky B, Orrenius S. The Warburg effect and mitochondrial stability in cancer cells. Mol Aspects Med. 2010;31:60–74. doi:10.1016/j.mam.2009.12.004. PMID:19995572
- Ruckenstuhl C, Büttner S, Carmona-Gutierrez D, et al. The Warburg effect suppresses oxidative stress induced apoptosis in a yeast model for cancer. PLoS One. 2009;4:e4592. doi:10.1371/journal.pone.0004592. PMID:19240798
- Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292:504–507. doi:10.1126/science.1058079. PMID:11283355
- Waddell TG, Repovic P, Meléndez-Hevia E, et al. Optimization of glycolysis: new discussions. Biochem Educ. 1999;27:12–13. doi:10.1016/S0307-4412(98)00266-0.
- MacLean RC, Gudelj I. Resource competition and social conflict in experimental populations of yeast. Nature. 2006;441:498–501. doi:10.1038/nature04624. PMID:16724064
- Wu L, Mashego MR, Proell AM, et al. In vivo kinetics of primary metabolism in Saccharomyces cerevisiae studied through prolonged chemostat cultivation. Metab Eng. 2006;8:160–171. doi:10.1016/j.ymben.2005.09.005. PMID:16233984
- Nilsson A, Nielsen J. Metabolic Trade-offs in Yeast are caused by F1F0-ATP synthase. Sci Rep. 2016;6:22264. doi:10.1038/srep22264. PMID:26928598
- Fonseca GG, Gombert AK, Heinzle E, et al. Physiology of the yeast Kluyveromyces marxianus during batch and chemostat cultures with glucose as the sole carbon source. FEMS Yeast Res. 2007;7:422–435. doi:10.1111/j.1567-1364.2006.00192.x. PMID:17233766
- Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer. 2016;16:635–649. doi:10.1038/nrc.2016.77. PMID:27634447
- Hui S, Silverman JM, Chen SS, et al. Quantitative proteomic analysis reveals a simple strategy of global resource allocation in bacteria. Mol Syst Biol. 2015;11:e784–e784. doi:10.15252/msb.20145697.
- Basan M, Hui S, Zhang Z, et al. Overflow metabolism in bacteria results from efficient proteome allocation for energy biogenesis. Nature. 2015;528:99–104. doi:10.1038/nature15765. PMID:26632588
- Madhukar NS, Warmoes MO, Locasale JW. Organization of enzyme concentration across the metabolic network in cancer cells. PLoS One. 2015;10.
- Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi:10.1146/annurev-cellbio-092910-154237. PMID:21985671
- Monod J. The growth of bacterial cultures. Annu Rev Microbiol. 1949;3:371–394. doi:10.1146/annurev.mi.03.100149.002103.
- Huberts DHEW, Niebel B, Heinemann M. A flux-sensing mechanism could regulate the switch between respiration and fermentation. FEMS Yeast Res. 2012;12:118–128. doi:10.1111/j.1567-1364.2011.00767.x. PMID:22129078
- Werner-Washburne M, Braun E, Johnston GC, et al. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1993;57:383–401. PMID:8393130
- Gray JV, Petsko GA, Johnston GC, et al. “Sleeping beauty”: quiescence in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2004;68:187–206. doi:10.1128/MMBR.68.2.187-206.2004. PMID:15187181
- Yoshimoto H, Ohuchi R, Ikado K, et al. Sugar induces death of the bottom fermenting yeast Saccharomyces pastorianus. J Biosci Bioeng. 2009;108:60–62. doi:10.1016/j.jbiosc.2008.12.022. PMID:19577194
- Santos J, Sousa MJ, Leão C. Ammonium is toxic for aging yeast cells, inducing death and shortening of the chronological lifespan. PLoS One. 2012;7:e37090. doi:10.1371/journal.pone.0037090. PMID:22615903
- Thevelein JM, Cauwenberg L, Colombo S, et al. Nutrient-induced signal transduction through the protein kinase A pathway and its role in the control of metabolism, stress resistance, and growth in yeast. Enzyme Microb Technol. 2000;26:819–825. doi:10.1016/S0141-0229(00)00177-0. PMID:10862891
- Crespo JL, Hall MN. Elucidating TOR signaling and rapamycin action: lessons from Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2002;66:579–591. doi:10.1128/MMBR.66.4.579-591.2002. PMID:12456783
- De Virgilio C, Loewith R. The TOR signalling network from yeast to man. Int J Biochem Cell Biol. 2006;38:1476–1481. doi:10.1016/j.biocel.2006.02.013. PMID:16647875
- Tsujimoto Y. Apoptosis and necrosis: intracellular ATP level as a determinant for cell death modes. Cell Death Differ. 1997;4:429–434. doi:10.1038/sj.cdd.4400262. PMID:16465263
- Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. PMID:9157970
- Proskuryakov S, Gabai V. Mechanisms of tumor cell necrosis. Curr Pharm Des. 2010;16:56–68. doi:10.2174/138161210789941793. PMID:20214618
- Kroemer G, Galluzzi L, Vandenabeele P, et al. Classification of cell death: recommendations of the nomenclature committee on cell death 2009. Cell Death Differ. 2009;16:3–11. doi:10.1038/cdd.2008.150. PMID:18846107
- Charles E, Hammadi M, Kischel P, et al. The antidepressant fluoxetine induces necrosis by energy depletion and mitochondrial calcium overload. Oncotarget. 2016;8:3181–3196.
- Bruce JIE. Metabolic regulation of the PMCA: role in cell death and survival. Cell Calcium. 2017;1–9.
- Sun W, Wu X, Gao H, et al. Cytosolic calcium mediates RIP1/RIP3 complex-dependent necroptosis through JNK activation and mitochondrial ROS production in human colon cancer cells. Free Radic Biol Med. 2017;108:433–444. doi:10.1016/j.freeradbiomed.2017.04.010. PMID:28414098
- Conrad M, Schothorst J, Kankipati HN, et al. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev. 2014;38:254–299. doi:10.1111/1574-6976.12065. PMID:24483210
- Gonzàlez A, Hall MN. Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 2017;36:2661–2807. doi:10.15252/embj.201696010. PMID:28871059
- Larsson C, Pahlman IL, Ansell R, et al. The importance of the glycerol 3-phosphate shuttle during aerobic growth of Saccharomyces cerevisiae. Yeast. 1998;14:347–357. doi:10.1002/(SICI)1097-0061(19980315)14:4%3c347::AID-YEA226%3e3.0.CO;2-9. PMID:9559543
- Oliveira GA, Tahara EB, Gombert AK, et al. Increased aerobic metabolism is essential for the beneficial effects of caloric restriction on yeast life span. J Bioenerg Biomembr. 2008;40:381–388. doi:10.1007/s10863-008-9159-5. PMID:18704665
- Kashiwaya Y, Sato K, Tsuchiya N, et al. Control of glucose utilization in working perfused rat heart. J Biol Chem. 1994;269:25502–25514. PMID:7929251
- Reijenga KA, Snoep JL, Diderich JA, et al. Control of glycolytic dynamics by hexose transport in Saccharomyces cerevisiae. Biophys J. 2001;80:626–634. doi:10.1016/S0006-3495(01)76043-2. PMID:11159431
- Feng W, Cui G, Tang C, et al. Role of glucose metabolism related gene GLUT1 in the occurrence and prognosis of colorectal cancer. Oncotarget. 2017;8:56850–56857. doi:10.18632/oncotarget.18090. PMID:28915636
- Youn WK, Park YK, Tae YY, et al. Expression of the GLUT1 glucose transporter in gallbladder carcinomas. Hepatogastroenterology. 2002;49:907–911. PMID:12143238
- Vaz CV., Marques R, Alves MG, et al. Androgens enhance the glycolytic metabolism and lactate export in prostate cancer cells by modulating the expression of GLUT1, GLUT3, PFK, LDH and MCT4 genes. J Cancer Res Clin Oncol. 2016;142:5–16. doi:10.1007/s00432-015-1992-4. PMID:26048031
- Barron CC, Bilan PJ, Tsakiridis T, et al. Facilitative glucose transporters: Implications for cancer detection, prognosis and treatment. Vol. 65. Metabolism: Clinical and Experimental; 2016. p. 124–139.
- Scafoglio C, Hirayama BA, Kepe V, et al. Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci U S A. 2015;112:E4111–E4119. doi:10.1073/pnas.1511698112. PMID:26170283
- Vander Heiden MG, Locasale JW, Swanson KD, et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi:10.1126/science.1188015. PMID:20847263
- Gombert AK, Dos Santos MM, Christensen B, et al. Network identification and flux quantification in the central metabolism of Saccharomyces cerevisiae under different conditions of glucose repression. J Bacteriol. 2001;183:1441–1451. doi:10.1128/JB.183.4.1441-1451.2001. PMID:11157958
- Jouhten P, Penttilä M. Anaerobic carbon metabolism of Saccharomyces cerevisiae. In: Pišku J, Compagno C, editors. Molecular mechanisms in yeast carbon metabolism. Berlin, Heidelberg: Springer; 2014. p. 57–82.
- Van Urk H, Voll WSL, Scheffers WA, et al. Transient-state analysis of metabolic fluxes in Crabtree-positive and crabtree-negative yeasts. Appl Environ Microbiol. 1990;56:281–287. PMID:16348101
- Hanson PJ, Parsons DS. The utilization of glucose and production of lactate by in vitro preparations of rat small intestine: effects of vascular perfusion. J Physiol. 1976;255:775–795. doi:10.1113/jphysiol.1976.sp011307. PMID:1263142
- Wang J, Wang H, Liu A, et al. Lactate dehydrogenase a negatively regulated by miRNAs promotes aerobic glycolysis and is increased in colorectal cancer. Oncotarget. 2015;6:19456–19468. PMID:26062441
- Häggström L, Ljunggren J, Öhman L. Metabolic engineering of animal cells. Ann NY Acad Sci. 1996;782:40–52. doi:10.1111/j.1749-6632.1996.tb40545.x. PMID:8659912
- Visser D, Van Zuylen GA, Van Dam JC, et al. Analysis of in vivo kinetics of glycolysis in aerobic Saccharomyces cerevisiae by application of glucose and ethanol pulses. Biotechnol Bioeng. 2004;88:157–167. doi:10.1002/bit.20235. PMID:15449293
- Bosch D, Johansson M, Ferndahl C, et al. Characterization of glucose transport mutants of Saccharomyces cerevisiae during a nutritional upshift reveals a correlation between metabolite levels and glycolytic flux. FEMS Yeast Res. 2008;8:10–25. doi:10.1111/j.1567-1364.2007.00323.x. PMID:18042231
- Peeters K, Van Leemputte F, Fischer B, et al. Fructose-1,6-bisphosphate couples glycolytic flux to activation of Ras. Nat Commun. 2017;8:922. doi:10.1038/s41467-017-01019-z. PMID:29030545
- Brambilla L, Bolzani D, Compagno C, et al. NADH reoxidation does not control glycolytic flux during exposure of respiring Saccharomyces cerevisiae cultures to glucose excess. FEMS Microbiol Lett. 1999;171:133–140. doi:10.1111/j.1574-6968.1999.tb13423.x. PMID:10077837
- Vemuri GN, Eiteman MA, McEwen JE, et al. Increasing NADH oxidation reduces overflow metabolism in Saccharomyces cerevisiae. Proc Natl Acad Sci. 2007;104:2402–2407. doi:10.1073/pnas.0607469104. PMID:17287356
- Agrimi G, Brambilla L, Frascotti G, et al. Deletion or overexpression of mitochondrial NAD+ carriers in Saccharomyces cerevisiae alters cellular NAD and ATP contents and affects mitochondrial metabolism and the rate of glycolysis. Appl Environ Microbiol. 2011;77:2239–2246. doi:10.1128/AEM.01703-10. PMID:21335394
- Stephanopoulos G. Metabolic fluxes and metabolic engineering. Metab Eng. 1999;1:1–11. doi:10.1006/mben.1998.0101. PMID:10935750
- Aon M, Cortassa S. Systems biology of the fluxome. Processes. 2015;3:607–618. doi:10.3390/pr3030607.
- Cortassa S, Caceres V, Bell LN, et al. From metabolomics to fluxomics: a computational procedure to translate metabolite profiles into metabolic fluxes. Biophys J. 2015;108:163–172. doi:10.1016/j.bpj.2014.11.1857. PMID:25564863
- Antoniewicz MR. Methods and advances in metabolic flux analysis: a mini-review. J Ind Microbiol Biotechnol. 2015;42:317–325. doi:10.1007/s10295-015-1585-x. PMID:25613286
- Forrester JW. System dynamics, systems thinking, and soft OR. Syst Dyn Rev. 1994;10:245–256. doi:10.1002/sdr.4260100211.
- Owen NA, Griffiths H. A system dynamics model integrating physiology and biochemical regulation predicts extent of crassulacean acid metabolism (CAM) phases. New Phytol. 2013;200:1116–1131. doi:10.1111/nph.12461. PMID:23992169
- Theobald U, Mailinger W, Baltes M, et al. In vivo analysis of metabolic dynamics in Saccharomyces cerevisiae: I. experimental observations. Biotechnol Bioeng. 1997;55:305–316. doi:10.1002/(SICI)1097-0290(19970720)55:2%3c305::AID-BIT8%3e3.0.CO;2-M. PMID:18636489
- Van Den Brink J, Canelas AB, Van Gulik WM, et al. Dynamics of glycolytic regulation during adaptation of Saccharomyces cerevisiae to fermentative metabolism. Appl Environ Microbiol. 2008;74:5710–5723. doi:10.1128/AEM.01121-08. PMID:18641162
