ABSTRACT
Objective: The present study was conducted to determine the role of gremlin during the development of posterior capsular opacification (PCO) via in vitro and in vivo experiments.
Methods: The activation, roles and relationships of the BMPs/Smad1/5, MAPK, FAK and AKT signaling pathways in human lens epithelial cells (HLECs) after gremlin induction were detected by western blotting and real-time PCR. Wound-healing, transwell, capsular bag models and rat PCO models assays were used to test the effects of gremlin on HLECs’ migration, proliferation, EMT-specific protein α-smooth muscle actin(α-SMA)and development of PCO in rats.
Results: Our data showed that knockdown of the gremlin inhibited the development of PCO and reduced expression of α-SMA in rats. While gremlin did not alter the migration of HLECs, it increased the expression of p-ERK and p-AKT. Knockout of Smad2 or Smad3 inhibited the expression of p-ERK and p-AKT proteins induced by gremlin. Gremlin also reduced BMP4-induced expression of the p-Smad1/5 protein. Finally, knockout of Smad1/5 increased gremlin-induced expression of α-SMA, fibronectin and type I collagen (COL-1) in HLECs.
Conclusion: These results suggested that gremlin contributed to the development of PCO by promoting LEC proliferation, activation of TGF-β/Smad, ERK and AKT signaling and inhibition of BMPs/Smad1/5 signaling. Furthermore, inhibiting gremlin effectively impaired both PCO development in rats and EMT in the lens capsule. Thus, our data suggest that gremlin might be a potential target for PCO.
1. Introduction
Posterior capsular opacification(PCO)is the most common postoperative complication after extracapsular cataract extraction or phacoemulsification surgery. The main causes of PCO are abnormal proliferation, transdifferentiation, and migration and production of collagen in residual lens epithelial cells (LECs) in the capsule [Citation1]. Our previous studies found that gremlin as a downstream mediator of TGF-β2, also plays an essential role in inducing epithelial mesenchymal transition (EMT)and extracellular matrix(ECM)protein production in human lens epithelial cells(HLECs)[Citation2].
Gremlin plays an important role in the development and homeostasis of many organs and in the pathogenesis of some fibrotic diseases [Citation3–Citation5]. Gremlin can effectively inhibit the activity of BMPs by binding to BMP extra- and intracellularly, thus blocking their binding to BMPRs. In addition, gremlin inhibits the activation of BMP signaling pathways by regulating the phosphorylation of Smad1/5/8 [Citation6–Citation8]. However, the effect of gremlin on the Smad1/5/8 signaling pathway and the mechanism by which gremlin induces EMT and ECM production in HLECs remains unknown.
TGF-β/Smad is considered the canonical signaling pathway of gremlin that mediates its biological function [Citation9–Citation11]. Fibronectin(Fn)and type I collagen (COL-1), in trabecular meshwork cells and the EMT-specific protein α-smooth muscle actin(α-SMA) [Citation9,Citation12]. Our previous studies showed that gremlin induced the expression of α-SMA, Fn and COL-1 in HLECs by activating the TGF-β/Smad2/3 signaling pathway [Citation2]. While other important signaling pathways, including AKT/mTOR, p38, JKN, ERK and WNT, also play important roles in the action of gremlin under many pathological and physiological conditions [Citation13–Citation16], their role in gremlin-induced EMT and ECM synthesis and their relationship with Smad2/3 in the process of gremlin-induced EMT and ECM synthesis are unclear.
HLEC proliferation and migration are the most important contributing factors to the development of PCO. Previous studies have suggested that gremlin promotes the proliferation and migration of vascular smooth muscle cells [Citation17] and tumor cells [Citation18]. However, the effect of gremlin on HLEC migration and proliferation is not known and so was studied herein.
Inhibition of gremlin suppresses fibrosis under various conditions, such as diabetic nephropathy, chronic pancreatitis and glaucomatous ocular hypertension [Citation19–Citation22]. However, it is currently unknown whether specific inhibition of gremlin can effectively inhibit the development of PCO.
In this work, a rat PCO model was first established to examine the impact of gremlin depletion on the development of PCO in vivo, based on previous reports of the effects of gremlin on EMT and ECM synthesis. Then, to investigate the potential mechanism, we studied the effect of gremlin on the proliferation and migration abilities of HLECs. Furthermore, we examined the expression of BMPs, BMPRs and components of the BMPs/Smad1/5 signaling pathways, as well as the role of gremlin in the activation of AKT, ERK, p38, JNK and FAK signaling pathways and its relationship with Smad2/3 signaling in HLECs.
2. Materials and methods
2.1. Culture and treatment of HLECs
The HLEC line B3 was purchased from ATCC (Manassas, VA, USA). A total of 1 × 106 cells within 12 passages were seeded into a culture flask with DMEM containing 10% foetal bovine serum (FBS). The culture medium was replaced with serum-free DMEM when the cells approached 70% confluence, and the cells were cultured for a further 24 h. The cells in the experimental group were then treated with 3 mL of serum-free medium containing gremlin at 200 μg/L(tested in our previous study [Citation2])and incubated for different lengths of time (0, 5, 15, 30 min and 1, 2, 4, 8, 16, 24 h) before cells were harvested for further analysis. Control group cells were treated with an equal volume of the medium since all of the reagents are water soluble.
2.2. Rat PCO model
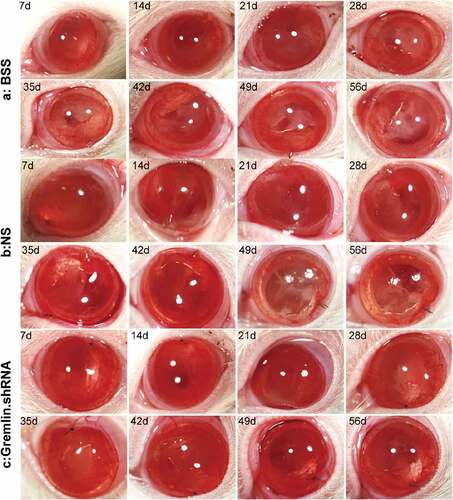
Thirty healthy adult SD rats (provided by Animal Experimentation Center of School of Medical of Xi’an Jiaotong University), with an average weight of 200 g and a sex ratio of 1:1, were selected for the experiment. Animal care, handling and surgical procedures were in accordance with the Animal Statement and Ethics Committee of Xi’an Jiaotong University Affiliated First Hospital. Pre-examination was conducted on the rats under a slit lamp to ensure that they were free from eye diseases, such as cornea infection or cataract. Extracapsular cataract extraction (ECCE) operations were performed on the left eye of each animal only. These rats were divided randomly into three groups: Group A (balanced salt solution (BSS) control), Group B (after surgery, 7 days after surgery and 14 days after surgery for three intracameral injections of NC shRNA), and Group C (after surgery, 7 days after surgery and 14 days after surgery for three intracameral injections of gremlin.shRNA-AAV complex). The target sequences for rat gremlin.shRNA and non-specific shRNA are shown in . The PCO model was created as described in our previous study [Citation23]. The formation of PCO was monitored daily using slit lamp microscopy. On the 60th day after surgery, the rats were sacrificed, and the globes were retrieved for further analysis.
Table 1. Target sequences of Smad2, Smad3, gremlin and non-sense control (NC) shRNA.
2.3. Immunohistochemical staining
Immunohistochemical staining was performed as described in our previous study [Citation24]. The primary antibodies used were: α-SMA (Cat no. ab5694, Abcam, Cambridge, MA, USA); gremlin (Cat no. 120–42, GeneTex, Irvine, California, USA); BMPRIA (Cat no. ab38560, Abcam, Cambridge, MA, USA); and BMPRII (Cat no. ab106266, Abcam, Cambridge, MA, USA). Other reagents used included secondary antibody include fluorescein-conjugated IgG (Alexa Fluor goat anti-rabbit IgG, Invitrogen) and Streptavidin-Biotin Complex (sp-9000, ZSGB-BIO, China).
2.4. Preparation of capsular bag model
Ten eyes of five pigs were obtained from an abattoir. The porn capsular bag model was created as described in our previous study [Citation25]. The capsular bags, which were treated with gremlin at 200 μg/L or control medium, were incubated in MEM medium (CM10010, M&C Gene Technology Ltd., China) for 14 days in an incubator of 5% CO2 at 38°C. The medium was changed every 48 h. LEC proliferation within capsules was observed with an inverted microscope (Eclipse Ti, Nikon, Japan). The experiments were repeated at least three times with each treatment.
2.5. Wound healing assay and transwell® migration assay
The transwell migration assay and Wound healing assay were performed to identify and evaluate the chemical cues of gremlin on cell migration as described in our previous study [Citation25]. Briefly, in the transwell assay, 104 cells were seeded in the upper chamber and serum-free medium with or without containing 200 μg/L gremlin was placed in the bottom compartment. 24 h later, cells were stained with crystal violet, and the migrated cells under the filter were counted. While in the Wound healing assay, 3 × 104 cells were seeded into the Culture-insert two well from Ibidi GmbH (Martinsried, Germany) which provided a 500 μm gap allowing cells to migrate from each side. After the insert was gently removed, cells were treated with or without 200 μg/L gremlin in serum-free medium. The images captured every 24 h were analyzed by Image J.
2.6. Quantitative real-time PCR and western blot
The total RNA extraction, reverse transcription, qPCR reaction and western blot were performed as described previously [Citation2]. The PCR primers were designed and synthesized by TaKaRa Biotechnology (TAKARA BIO Inc., Japan), as shown in . The antibodies used in the western blot are shown in .
Table 2. PCR primers used in the study.
Table 3. List of antibodies used for western blot.
2.7. Transfection of HLECs with shRNA
Recombinant lentiviruses expressing Smad2 and Smad3 small interfering RNA of (Smad2.shRNA and Smad3.shRNA, respectively) were purchased from Neuron Biotech Co., Ltd. (Shanghai, China). The target sequences for Smad2.shRNA, Smad3.shRNA and non-specific control shRNA (NC.shRNA) are shown in . The cells were cultured in a six-well plate at 2 × 105 cells/well for 12 h. Then, the medium was removed, and the cells were washed with PBS before adding serum-free medium containing 5 μL of lentivirus (4 × 106 virus, MOI = 1:20). The cells were further incubated for 24 h before the medium was replaced with 2 mL of DMEM containing 10% FBS and cultured for a further 24 h.
Table 4. PCO grade in different groups of rats at 56 days after surgery.
2.8. Anterior chamber reaction and PCO grading
Rat PCO grading was performed by slit-lamp biomicroscopy at 60 days after surgery, as shown in . We also evaluated cellular inflammation in the anterior chamber after surgery, according to the number of inflammatory cells and fibrinous exudate in the anterior chamber: none: a clear aqueous humour or a few floating cells (6–15 cells) in the anterior chamber; mild: a few cells (16–25 cells) in the anterior chamber; moderate: many cells (26–50 cells) and some fibrinous exudate in the anterior chamber; and severe: a very large number of cells (>50 cells) and a large amount of fibrinous exudate in the anterior chamber. The grading of anterior chamber inflammation in all three groups is shown in . Inflammation in the anterior chamber was severe after surgery and became increasingly mild over time.
Table 5. Reaction in the anterior chamber after surgery.
2.9. Statistical analysis
GraphPad Prism statistics software, version 5.0, was employed to perform all of the statistical analyses. All of the data are presented as the mean ± SD from at least three independent in vitro experiments. Statistical comparisons of transwell, real-time PCR and western blot data with those of the control group were performed using one way ANOVA, while the difference between rat groups in the PCO model was compared using Tukey’s Honest Significant Difference test. A p value <0.05 was considered statistically significant.
3. Results
3.1. Specific silencing of gremlin inhibits PCO development in rats
Our previous studies showed that gremlin is a downstream mediator of TGF-β2, an important player in the pathogenesis of PCO and other fibrotic diseases in various tissues [Citation10,Citation14,Citation26]. Therefore, we hypothesized that targeting gremlin could delay the onset of PCO or alleviate its symptoms. To test this hypothesis, we enrolled in an in vivo rat PCO model which described in detail in our previous study [Citation25]. In short, after rats were anesthetized using sodium pentobarbital and pupils diluted, viscoelastic was injected to form the anterior chamber from the 11-point corneal 2.0 mm cut. Then, a discission needle performed the capsulectomy and hydrodissection was done to remove the cortex and nuclear. we injected gremlin.shRNA with adeno-associated virus (AAV) vector to silence the expression of gremlin and observe its effect on PCO. The results showed that the opacification started to present as a grey-white band or plaque-like opacity in the negative control virus group after 28 days, and the degree of opacification was increased in these animals on day 49 after surgery (,)). However, the capsules of the rats that received intracameral injections of gremlin.shRNA were still transparent on day 56 ()).
3.2. Assessment of PCO
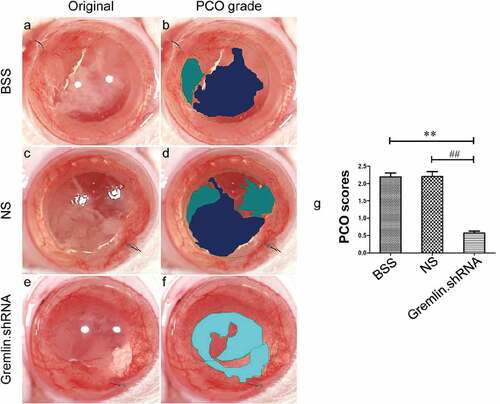
The level of opacification in the eyes of all three groups was further analyzed using EPCO software (http://www.epco2000.de/). The PCO grades were evaluated as shown in . The results showed that rats treated with gremlin.shRNA AAV had significantly lower PCO scores than rats from the BSS and NS groups ( ##p < 0.01, **p < 0.01). Together, our results indicated that specific blockade of gremlin could effectively delay and inhibit the occurrence of PCO.
Table 6. PCO grading standard.
Figure 2. Comparison of PCO scores by EPCO software. The PCO scores from the BSS group (a and b), NS group (c and d) and gremlin.shRNA group (e and f) were compared using the EPCO software program. One way ANOVA was used for statistical analyses and significant differences were observed in the PCO scores between the BSS and gremlin.shRNA AAV groups (**p < 0.01) and between the NS and gremlin.shRNA AAV groups (##p < 0.01).
3.3. Expression of α-SMA and gremlin proteins in the lens anterior and posterior capsules
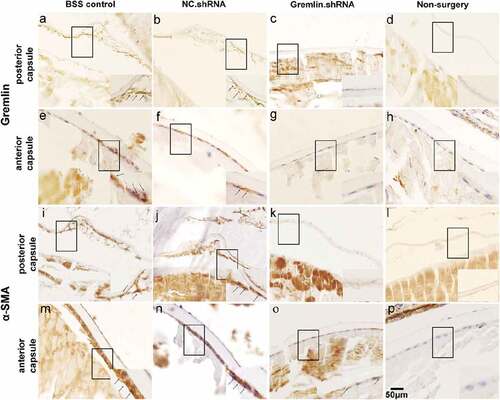
To verify the effects of gremlin on PCO development and EMT proteins, similar experiments were performed with surgery and knockdown of gremlin by shRNA AAV. Rats were sacrificed on day 56 after the operation, and the cells and fibers in the posterior capsule and the expression of α-SMA and gremlin protein in the anterior and posterior lens capsules were examined using IHC. The results showed that in both the BSS and NS groups, there were numerous migrated LECs and fibers under the posterior capsule. These cells had lost their primary morphology and changed from being fibroblast like monolayered and polygonal shaped to being tabular shaped, multi-layered and irregularly arranged (,,,)), while the number of migrated LECs and fibers was decreased in gremlin.shRNA-treated animals (,)). At the same time, the expression of α-SMA protein in the anterior capsule was also significantly reduced in the eyes of gremlin.shRNA-treated rats compared with that in BBS and NS control rats (,,)). We also observed gremlin expression in the LECs of the BSS control and NS groups (,)). After gremlin.shRNA treatment, the expression of gremlin decreased significantly in the LECs ()). As expected, we did not detect migrating LECs, collagenous fibers, and α-SMA and gremlin protein in the non-surgery group (,,,)). These results indicated that the gremlin-specific shRNA successfully blocked the surgery-induced upregulation of LEC migration, collagenous fiber formation under the posterior capsule and α-SMA protein expression in the anterior capsule.
Figure 3. Expression of gremlin and α-SMA protein in the lens capsule, as detected by immunohistochemistry (IHC). Expression of gremlin in the posterior capsule and anterior capsule of the BSS group (a and e), NS group (b and f), gremlin.shRNA group (c and g) and non-surgery group (d and h). Expression of α-SMA in the posterior capsule and anterior capsule of the BSS group (i and m), NS group (j and n), gremlin.shRNA group (k and o) and non-surgery group (l and p). Scale bar: 50 µm.
3.4. Gremlin does not affect the migration capability of HLECs
We first investigated the impact of gremlin on the migration ability of HLECs using transwell and wound-healing assays. The results showed that the number of migrated cells was similar in the cell groups with or without treatment with 200 μg/L gremlin for 24 h (Figure S1a, b, c). Similar results were also observed in the wound-healing assay (Figure S1 d, e, f, g, h, i, j). The results indicated that gremlin does not affect the migration capability of HLECs.
3.5. Gremlin promotes LEC proliferation and induces the production of the EMT-specific protein α-SMA in a capsular bag model
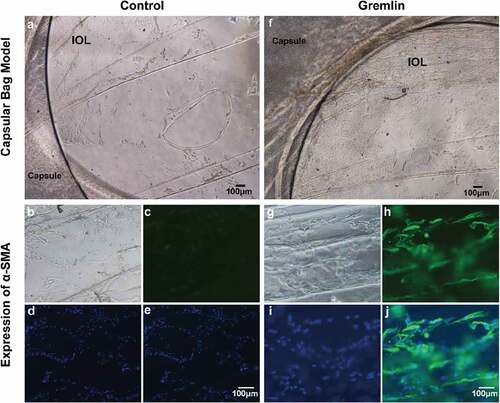
Proliferation and migration of the remaining cells in the capsule after cataract surgery are considered the important procedure in the formation of PCO and ex vivo PCO model is a simply effective way to study the dynamic process of cell behavior like proliferation, migration and EMT. So we next investigated the impact of gremlin on the EMT and proliferation ability of LECs using a capsular bag model. Capsular bags were carefully prepared from pig eyes and filled with medium with or without gremlin. After 14 days, the number of LECs in the gremlin-treated (f)) capsular bags were markedly increased compared with that in the control capsular bags ()). Furthermore, immunofluorescence staining indicated that the expression of α-SMA was increased in the capsular bag after gremlin treatment ()) compared with that in the control capsular bag ()). The results suggested that gremlin induces EMT-specific protein expression and proliferation in LECs in the capsular bag model.
Figure 4. Gremlin promotes LEC proliferation and induces EMT-specific protein expression in a capsular bag model. A capsular bag model was established and cultured with or without gremlin. (a) Control group on day 14; (b, c, d, e) The expression of α-SMA in capsular bags in the control group; (f) Capsular bags that were cultured with 200 μg/L gremlin on day 14; (g, h, i, j) Expression of α-SMA in capsular bags after treatment with gremlin. (b, g) Natural light; (c, h) IHC of α-SMA; (d, i) DAPI staining; (e, j) Merged. The experiments were repeated three times with five samples in each group each time.
3.6. The effect of gremlin on the expression of BMPRs and BMPs in HLECs
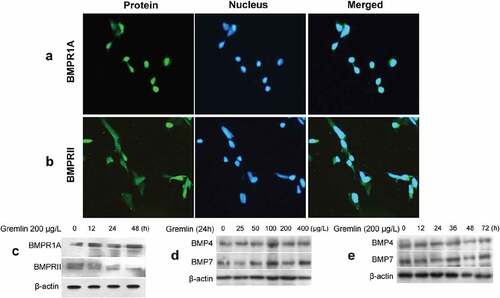
Gremlin is one of the major endogenous antagonists of BMPs [Citation27,Citation28]. While BMP signaling is transduced via binding to BMPRs especially the BMPRIA and BMPRII heterodimer generally for BMP4 and BMP7 [Citation29,Citation30]. We next investigated the effect of gremlin on the expression levels of BMPs and BMPRs in HLECs. The results showed that HLECs express BMPRIA and BMPRII both in the nucleus and the cytoplasm. In particular, the majority of them distributed in the nucleus rather than the cytoplasm (,)). After treatment with 200 μg/L gremlin for 12, 24 and 48 h, the expression of BMPRIA was increased obviously compared with controls and presented in a time-dependent manner. However, in contrast, the expression of BMPRII was reduced (),Figure S2a). After treatment with different concentrations of gremlin (0, 25, 50, 100, 200 and 400 μg/L) for 24 h, the BMP4 and BMP7 proteins were not significantly changed (), Figure S2b). Furthermore, after treatment with 200 μg/L gremlin for different lengths of time (0, 12, 24, 36, 48 and 72 h), the expression of BMP4 and BMP7 proteins was also not significantly changed (), Figure S2c).
Figure 5. Effect of gremlin on the expression of BMPRs and BMPs in HLECs. (a, b) BMPRIA and BMPRII expression in HLECs. HLECs were treated with different concentrations of gremlin or with 200 μg/L gremlin for different lengths of time. (c) Detection of BMPRIA and BMPRII expression; (d) Detection of BMP4 protein expression. (e) Detection of BMP7 protein expression.
3.7. The role of the BMP/Smad1/5 signaling pathway in gremlin-induced EMT and ECM synthesis in HLECs
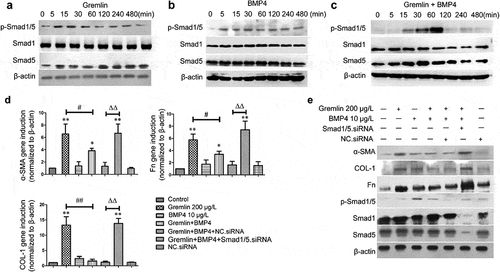
Inhibition of BMPs/Smad1/5 signaling is considered an important mechanism of gremlin-induced fibrosis [Citation7,Citation8]. We then studied the involvement of this signaling pathway in gremlin-induced EMT and ECM production in HLECs. Our results showed that gremlin treatment increased the expression of p-Smad1/5 protein after 5 and 15 min but reduced its expression rapidly from 30 min, after which p-Smad1/5 protein levels remained stable at a low level (), Figure S3a). Similarly, BMP4 increased the expression of p-Smad1/5 protein (), Figure S3b). While gremlin suppressed the BMP4 induced increase in p-Smad1/5 expression after 120 min (), Figure S3c). In addition, gremlin could increase the expression of α-SMA, Fn and COL-1, but this effect could be inhibited by BMP4. After blocking Smad1/5 signaling pathway by siRNA, the suppression of α-SMA, Fn and COL-1 expression by BMP4 was reversed (,), Figure S3d). Taken together, gremlin increased α-SMA, Fn and COL-1 by suppressing Bmp4 activity, which would otherwise suppress α-SMA, Fn and COL-1 expression, therein Smad1/5 signaling played key roles.
Figure 6. Effects of Gremlin on the levels of Bmp/Smad1/5. (a, b, c) Western blot assay was used to detect the expression of p-Smad1/5, Smad1 and Smad5 proteins induced by 200 μg/L gremlin, 10 μg/L BMP4 and 200 μg/L gremlin+10 μg/L BMP4 for different durations. (d, e) Real-time PCR and western blot assay were used to detect the expression of α-SMA, Fn and COL-1 following treatment with gremlin, BMP4, gremlin+BMP4, and gremlin+BMP4+ Smad1/5.siRNA.
3.8. The roles of AKT, ERK and Smad2/3 signaling pathways in the function of gremlin in inducing EMT and ECM synthesis in HLECs
Several studies have demonstrated that gremlin exerts its biological effects by activating TGF-β/Smad, AKT, ERK and other signaling pathways [Citation16,Citation31]. Our previous studies demonstrated that gremlin could dose-dependently activate the Smad2/3 signaling pathway. Here, we investigated whether these signaling pathways were activated by gremlin in HLECs and whether these pathways contributed to PCO development. HLECs were treated with 200 μg/L gremlin for different lengths of time (0, 5, 15, 30, 60, 120, 240 and 480 min); then, the expression of p-Smad2, Smad2, p-Smad3, Smad3, p-AKT, AKT, p-ERK1/2, ERK1/2, p-p38, p38, p-JNK, JNK, p-FAK and FAK was detected by western blotting. The results indicated that gremlin treatment increased the expression of p-Smad2, p-Smad3, p-AKT and p-ERK1/2 in a time-dependent manner, while the protein levels of total Smad2, Smad3, AKT and ERK1/2 were not changed (,b), Figure S4a, b). In contrast, there were no notable changes in the protein expression levels of p-p38, p38, p-JNK, JNK, p-FAK or FAK (), Figure S4c). Thus, the data suggested that gremlin could activate HLECs through the TGF-β/Smad, AKT and ERK signaling pathways.
Figure 7. Effects of Gremlin on the levels of AKT, ERK and Smad2/3. HLECs were treated with 200 μg/L gremlin for different lengths of time (0, 5, 15, 30, 60, 120, 240 and 480 min), (a) Detection of p-Smad2, Smad2, p-Smad3 and Smad3 protein expression by western blot; (b) Detection of p-ERK1/2, ERK1/2, p-AKT and AKT protein expression by western blot; (c) p-p38, p38, p-FAK and FAK protein expression as detected by western blot. Effects of the MEK inhibitor U0126 on gremlin-induced expression of α-SMA, Fn, COL-1, p-AKT, AKT, p-ERK and ERK in HLECs. (d) mRNA levels of α-SMA, Fn and COL-1 in HLECs treated with or without gremlin and/or LY294002; ** p < 0.01 versus the controls; ##p < 0.01 versus the group treated with gremlin or gremlin+LY294002. (f) Protein expression levels of α-SMA, Fn, COL-1, p-AKT, AKT, p-ERK and ERK in HLECs treated with or without gremlin and/or LY294002. (e) mRNA levels of α-SMA, Fn and COL-1 in HLECs treated with or without gremlin and/or U0126; *p < 0.05 and **p < 0.01 versus the controls; ##p < 0.01, statistically significant difference between the group treated with gremlin and the group treated with gremlin+U0126. (g) Protein expression levels of α-SMA, Fn, COL-1, p-ERK, ERK, p-AKT and AKT in HLECs treated with or without gremlin and/or U0126. (h) Protein expression levels of p-AKT, AKT, p-ERK and ERK in HLECs treated with or without gremlin and/or Smad2.shRNA. (i) Protein expression levels of p-AKT, AKT, p-ERK and ERK in HLECs treated with or without gremlin and/or Smad3.shRNA.
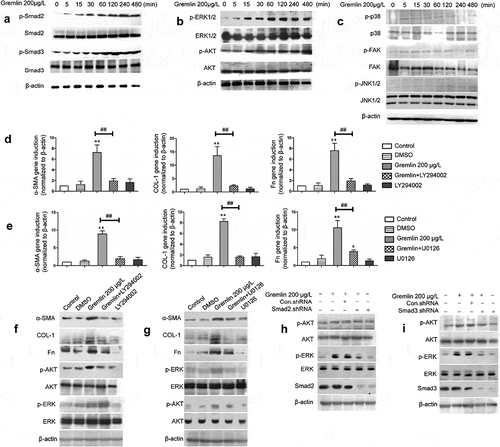
To further understand whether the AKT and ERK signaling pathways are involved in gremlin-induced expression of EMT markers and ECM proteins in HLECs, cells were treated with LY294002 and U0126 to block the AKT and ERK signaling pathways, respectively, followed by treatment with gremlin for 24 h. While the expression of α-SMA, Fn and COL-1 mRNA and protein was increased significantly after treatment with 200 μg/L gremlin compared with that in the control group, as expected, this increase was blocked by the AKT and ERK inhibitors LY294002 and U0126, respectively (,,,), Figure S5; *p <0.05, **p <0.01, ##p <0.01). Furthermore, the expression of p-ERK protein was not changed after blocking the AKT signaling pathway by LY294002 (), Figure S5a). Similarly, the expression of p-AKT protein was not changed after blocking of the ERK signaling pathway by U0126 (), Figure S5b).
The signaling pathways of TGF-β/Smad [Citation9,Citation10], AKT and ERK all play important roles in the induction of EMT and ECM production in HLECs by gremlin. Thus, we further examined the relationship among the AKT, ERK and TGF-β/Smad signaling pathways. HLECs were treated with Smad2.shRNA and Smad3.shRNA to block the TGF-β/Smad signaling pathway, followed by treatment with gremlin for 120 min. The data showed that the gremlin-induced increase in the expression of p-AKT and p-ERK protein was significantly inhibited by blocking the TGF-β/Smad signaling pathway with Smad2.shRNA and Smad3.shRNA compared with the controls (**p < 0.01) (,i), Figure S6). Interestingly, the expression levels of p-AKT and p-ERK protein remained significantly higher in the gremlin+Smad2.shRNA or gremlin+Smad3.shRNA group than in the control group.
4 Discussion
Cell proliferation, migration, transdifferentiation and collagen synthesis in the capsule after cataract surgery are the main causes of PCO [Citation1,Citation32,Citation33].
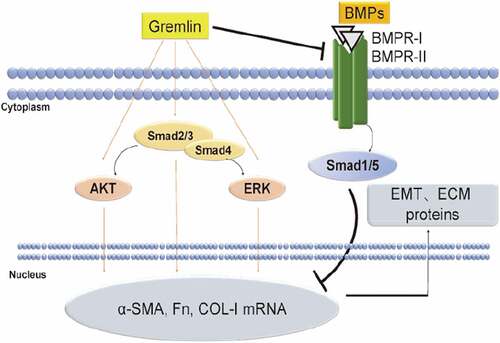
Herein we proposed a possible mechanism of gremlin function in preventing PCO: cataract surgery activates originally TGF-β2 precursor in the aqueous humor and lens. TGF-β2 further induces the expression of gremlin, which suppressed the expression of protective proteins, such as BMPs, and eventually activates the Smad2/3, AKT and ERK signaling pathways, thereby inducing transdifferentiation of LECs into fusiform myofibroblasts. Gremlin further promotes ECM synthesis, leading to the formation of plaque-like aggregation and excessive ECM protein production and accumulation, finally contributed to the development of PCO (). Taken together, we elucidated a potential mechanistic basis between gremlin and PCO, which highlighted the regulatory factors underlying the pathogenesis of fibrosis disease.
Figure 8. Schematic diagram of the proposed mechanism by which gremlin regulates EMT and ECM protein expression in LECs. The expression level of gremlin was increased in the aqueous humor after cataract surgery. Gremlin promoted EMT and ECM synthesis by activating the Smad2/3, ERK and AKT signaling pathways and suppressing the BMPs/Smad1/5 signaling pathway. Accumulation of EMT and ECM proteins led to the development of PCO.
Previous reports have suggested that gremlin could promote RPE cells and fibroblast-like synoviocytes migration and proliferation [Citation16,Citation34] while Helena showed that gremlin had no effect on RPE cell migration [Citation12]. Our data here showed that gremlin did not change the migratory capacity of HLECs in vitro but significantly promoted LECs proliferation together with increased levels of α-SMA protein in the capsular bag compared with those in the control group. For the antiproliferation effects of BMPs[Citation35], gremlin could no surprise enhance cell proliferation. However, gremlin did not influence cell migration in vitro according to our data. Whether gremlin has effects on HLECs directly or indirectly in vivo in PCO need further study.
Our results showed that gremlin could inhibit BMPs/Smad1/5 signaling, which in turn could effectively enhance EMT and ECM synthesis in HLECs. Some researchers have found that gremlin could promote EMT and ECM synthesis in trabecular meshwork (TM) cells and idiopathic pulmonary fibrosis via Smad1/5 signaling inhibiting [Citation7,Citation8]. These results indicated that inhibition of BMPs/Smad1/5 signaling played an important role in gremlin-induced EMT and ECM synthesis in HLECs.
The AKT, ERK, p38, JNK and FAK signaling pathways are involved in EMT and ECM production in a variety of cells [Citation36,Citation37]. Our studies showed that gremlin activates the Smad2/3 signaling pathway in a dose- and time-dependent manner [Citation2]. Smad2/3 [Citation9,Citation12] and the AKT and ERK signaling pathways play important roles in gremlin exerting its biologic effects. The results showed that blocking the Smad2/3 signaling pathway could inhibit the AKT and ERK signaling pathways activated by gremlin. Interestingly, although blocking the Smad2/3 signaling pathway could inhibit gremlin and activate the AKT and ERK signaling pathways, the expression of p-AKT and p-ERK proteins remained significantly enhanced compared with that in the controls. These results might indicate that part of the effects of the AKT and ERK signaling pathways in gremlin-induced EMT and ECM synthesis in HLECs were dependent on activation of the Smad2/3 signaling pathway. Moreover, the Smad2/3 signaling pathway could be the central link in regulating the process of gremlin-induced EMT and ECM synthesis in HLECs.
Gremlin could antagonize the antiproliferative effect of BMPs by binding to BMPs and preventing them from interacting with their receptors (like BMPRIA) [Citation12,Citation35]. Consistently, our study detected increased BMPRIA protein expression with BMP4 and BMP7 proteins unchanged in HLECs after treatment with gremlin, suggesting that gremlin could enhance the BMPRIA activity in both protein and phosphorylation level by preventing the binding with their BMP receptors. Intriguingly, we also detected modest decrease of BMPRII expression. We speculated that a feedback loop involving some other factors might play roles in repressing BMPRII expression to buffer and stabilize the excessive activity of BMPRIA. Further thorough investigations are required to decipher the precise regulatory mechanisms.
Therefore, inhibiting the function of gremlin might effectively prevent the development of PCO after cataract surgery.
Supplemental Material
Download MS Word (21.6 MB)Acknowledgments
We thank the Institute of Neurobiology of the Xi’an Jiaotong University College of Medicine for assisting in the study. All authors approved the final version of the manuscript.his work was supported by the National Natural Science Foundation of China [81470614]; National Natural Science Foundation for Young Scientists of China [81470614]; The Fundamental Research Funds for the Central Universities [xjj2018099]; The Natural Science Foundation of Shaanxi Province [No. 2018JQ8021]; The First Affiliated Hospital of Xi’an Jiaotong University Foundation [2016QN-04]; China Postdoctoral Science Foundation[2018 M633528].
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Wormstone IM, Wang L, Liu CS. Posterior capsule opacification. Exp Eye Res. 2009;88:257–269.
- Ma B, Kang Q, Qin L, et al. TGF-beta2 induces transdifferentiation and fibrosis in human lens epithelial cells via regulating gremlin and CTGF. Biochem Biophys Res Commun. 2014;447:689–695.
- Costello CM, Cahill E, Martin F, et al. Role of gremlin in the lung: development and disease. Am J Respir Cell Mol Biol. 2010;42:517–523.
- Nishinakamura R, Sakaguchi M. BMP signaling and its modifiers in kidney development. Pediatr Nephrol. 2014;29:681–686.
- Koli K, Sutinen E, Ronty M, et al. Gremlin-1 overexpression in mouse lung reduces silica-induced lymphocyte recruitment - a link to idiopathic pulmonary fibrosis through negative correlation with CXCL10 chemokine. PLoS One. 2016;11:e0159010.
- Sun J, Zhuang FF, Mullersman JE, et al. BMP4 activation and secretion are negatively regulated by an intracellular gremlin-BMP4 interaction. J Biol Chem. 2006;281:29349–29356.
- Yeung CY, Gossan N, Lu Y, et al. Gremlin-2 is a BMP antagonist that is regulated by the circadian clock. Sci Rep. 2014;4:5183.
- Staloch D, Gao X, Liu K, et al. Gremlin is a key pro-fibrogenic factor in chronic pancreatitis. J Mol Med (Berl). 2015;93:1085–1093.
- Sethi A, Jain A, Zode GS, et al. Role of TGFbeta/Smad signaling in gremlin induction of human trabecular meshwork extracellular matrix proteins. Invest Ophthalmol Vis Sci. 2011;52:5251–5259.
- Li G, Li Y, Liu S, et al. Gremlin aggravates hyperglycemia-induced podocyte injury by a TGFbeta/smad dependent signaling pathway. J Cell Biochem. 2013;114:2101–2113.
- Guan Y, Cheng W, Zou C, et al. Gremlin1 promotes carcinogenesis of glioma in vitro. Clin Exp Pharmacol Physiol. 2017;44:244–256.
- Lee H, O’Meara SJ, O’Brien C, et al. The role of gremlin, a BMP antagonist, and epithelial-to-mesenchymal transition in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2007;48:4291–4299.
- Heinke J, Juschkat M, Charlet A, et al. Antagonism and synergy between extracellular BMP modulators Tsg and BMPER balance blood vessel formation. J Cell Sci. 2013;126:3082–3094.
- Sethi A, Wordinger RJ, Clark AF. Gremlin utilizes canonical and non-canonical TGFbeta signaling to induce lysyl oxidase (LOX) genes in human trabecular meshwork cells. Exp Eye Res. 2013;113:117–127.
- Wu Q, Tang SG, Yuan ZM. Gremlin 2 inhibits adipocyte differentiation through activation of Wnt/beta-catenin signaling. Mol Med Rep. 2015;12:5891–5896.
- Liu Y, Chen Z, Cheng H, et al. Gremlin promotes retinal pigmentation epithelial (RPE) cell proliferation, migration and VEGF production via activating VEGFR2-Akt-mTORC2 signaling. Oncotarget. 2017;8:979–987.
- Maciel TT, Melo RS, Schor N, et al. Gremlin promotes vascular smooth muscle cell proliferation and migration. J Mol Cell Cardiol. 2008;44:370–379.
- Kim M, Yoon S, Lee S, et al. Gremlin-1 induces BMP-independent tumor cell proliferation, migration, and invasion. PLoS One. 2012;7:e35100.
- Church RH, Ali I, Tate M, et al. Gremlin1 plays a key role in kidney development and renal fibrosis. Am J Physiol Renal Physiol. 2017;312:F1141–f57.
- McDowell CM, Hernandez H, Mao W, et al. Gremlin induces ocular hypertension in mice through Smad3-dependent signaling. Invest Ophthalmol Vis Sci. 2015;56:5485–5492.
- Rastellini C, Han S, Bhatia V, et al. Induction of chronic pancreatitis by pancreatic duct ligation activates BMP2, apelin, and PTHrP expression in mice. Am J Physiol Gastrointest Liver Physiol. 2015;309:G554–65.
- Zhang Q, Shi Y, Wada J, et al. In vivo delivery of Gremlin siRNA plasmid reveals therapeutic potential against diabetic nephropathy by recovering bone morphogenetic protein-7. PLoS One. 2010;5:e11709.
- Ma B, Yang L, Jing R, et al. Effects of Interleukin-6 on posterior capsular opacification. Exp Eye Res. 2018;172:94–103.
- Zhang Z, Hu F, Liu Y, et al. Activation of type 5 metabotropic glutamate receptor promotes the proliferation of rat retinal progenitor cell via activation of the PI-3-K and MAPK signaling pathways. Neuroscience. 2016;322:138–151.
- Ma B, Jing R, Liu J, et al. CTGF contributes to the development of posterior capsule opacification: an in vitro and in vivo study. Int J Biol Sci. 2018;14:437–448.
- Rodrigues-Diez R, Lavoz C, Carvajal G, et al. Gremlin is a downstream profibrotic mediator of transforming growth factor-beta in cultured renal cells. Nephron Exp Nephrol. 2012;122:62–74.
- Rider CC, Heparin MB. Heparan sulphate and the TGF-beta cytokine superfamily. Molecules. 2017;22:713.
- Tatsinkam AJ, Rune N, Smith J, et al. The binding of the bone morphogenetic protein antagonist gremlin to kidney heparan sulfate: such binding is not essential for BMP antagonism. Int J Biochem Cell Biol. 2017;83:39–46.
- Burton VJ, Ciuclan LI, Holmes AM, et al. Bone morphogenetic protein receptor II regulates pulmonary artery endothelial cell barrier function. Blood. 2011;117:333–341.
- Kausar T, Nayeem SM. Correlating interfacial water dynamics with protein-protein interaction in complex of GDF-5 and BMPRI receptors. Biophys Chem. 2018;240:50–62.
- Bylund JB, Trinh LT, Awgulewitsch CP, et al. Coordinated proliferation and differentiation of human-induced pluripotent stem cell-derived cardiac progenitor cells depend on bone morphogenetic protein signaling regulation by GREMLIN 2. Stem Cells Dev. 2017;26:678–693.
- Taiyab A, Korol A, Deschamps PA, et al. beta-Catenin/CBP-Dependent Signaling Regulates TGF-beta-Induced Epithelial to Mesenchymal Transition of Lens Epithelial Cells. Invest Ophthalmol Vis Sci. 2016;57:5736–5747.
- Boswell BA, Korol A, West-Mays JA, et al. Dual function of TGFbeta in lens epithelial cell fate: implications for secondary cataract. Mol Biol Cell. 2017;28:907–921.
- Han EJ, Yoo SA, Kim GM, et al. GREM1 is a key regulator of synoviocyte hyperplasia and invasiveness. J Rheumatol. 2016;43:474–485.
- Pang K, Ryan JF, Baxevanis AD, et al. Evolution of the TGF-beta signaling pathway and its potential role in the ctenophore, Mnemiopsis leidyi. PLoS One. 2011;6:e24152.
- Huang M, Wang YP, Zhu LQ, et al. MAPK pathway mediates epithelial-mesenchymal transition induced by paraquat in alveolar epithelial cells. Environ Toxicol. 2016;31:1407–1414.
- Yang J, Hou Y, Zhou M, et al. Twist induces epithelial-mesenchymal transition and cell motility in breast cancer via ITGB1-FAK/ILK signaling axis and its associated downstream network. Int J Biochem Cell Biol. 2016;71:62–71.