ABSTRACT
The spindle constitutes the cellular machinery that enables the segregation of the chromosomes during eukaryotic cell division. The microtubules that form this fascinating and complex genome distribution system emanate from specialized structures located at both its poles and known as microtubule-organizing centers (MTOCs). Beyond their structural function, the spindle MTOCs play fundamental roles in cell cycle control, the activation and functionality of the mitotic checkpoints and during cellular aging. This review highlights the pivotal importance of spindle-associated MTOCs in multiple cellular processes and their central role as key regulatory hubs where diverse intracellular signals are integrated and coordinated to ensure the successful completion of cell division and the maintenance of the replicative lifespan.
Introduction
The spindle is a remarkable and complex cellular machinery that facilitates the equal distribution of the duplicated genome during cell division and the fidelity of this vital process [Citation1, Citation2]. It is constituted by a bipolar array of microtubules that provide structural support and that, together with microtubule-associated and motor proteins, guide the segregation of chromosomes. The microtubules from the spindle are nucleated from microtubule-organizing centers (MTOCs), located at both its poles, and known as the centrosomes in higher eukaryotes or the spindle pole bodies (SPBs) in yeast (). The structure and role of these MTOCs in microtubule nucleation has been extensively studied and analyzed [Citation1,Citation3,Citation4]. However, the MTOCs have been also shown to act as platforms where key components of essential signaling pathways allocate to facilitate the accurate positioning of the spindle, the proper and timely regulation of cell cycle progression, the coordination of this process with cell metabolism, and the functionality of the mitotic checkpoints. These structures can be further used by the cells to enable the distribution of proteins as well as other cellular components. Moreover, MTOCs can facilitate the differential segregation of molecules and organelles between the mother and daughter cells, thereby also representing a key mechanism to generate asymmetry during cell division. This review aims to highlight all these additional functions that turn MTOCs into the epicenter for the integration and coordination of fundamental cellular processes.
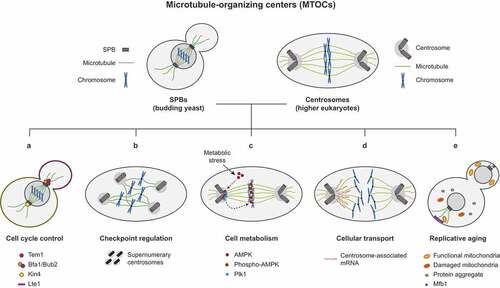
Figure 1. The multiple roles of microtubule organizing centers in the cell. The spindle pole bodies (SPBs) and centrosomes are the microtubule-organizing centers (MTOCs) that orchestrate spindle formation in yeast and higher eukaryotes, respectively. Besides their structural function, and among others, these fascinating structures also play important roles in (A) the regulation of cell cycle entry and progression, (B) the functionality of the mitotic checkpoints, (C) the interplay between the cell cycle machinery and the nutrient sensing pathways, (D) the differential transport of molecules and organelles and (E) during replicative aging.
The MTOCs in the regulation of cell cycle progression
Centrosomes and SPBs are key platforms where different cell cycle signaling pathways converge. The localization of essential cell cycle regulators to the spindle MTOCs is of pivotal importance for a timely and spatial coordination of many processes that ensure the correct progression through mitosis and the faithful distribution of the genome and cellular components between the mother and daughter cells. This is exemplified in the budding yeast Saccharomyces cerevisiae, in which localization of the Tem1 GTPase to SPBs is required for a successful completion of mitosis () [Citation5]. Tem1 initiates the mitotic exit network (MEN), a signaling pathway most of which components localize to the SPBs [Citation6]. From these structures, the MEN transduces a signal that facilitates the release throughout the cell of the Cdc14 phosphatase, which is sequestered in the nucleolus during most of the cell cycle, at anaphase onset [Citation5–Citation9]. Once released, Cdc14 promotes the global inactivation of cyclin-dependent kinase (CDK) activity, the main driver of cell cycle progression, thereby promoting the final events of mitosis [Citation7,Citation8]. Remarkably, research in our group recently demonstrated that artificial tethering of Tem1 away from the SPBs prevents mitotic exit in budding yeast, supporting that localization of MEN proteins to these structures is an essential requirement for normal cell cycle progression [Citation5]. Similarly, simultaneous laser ablation of both SPBs in Schizosaccharomyces pombe inhibits cytokinesis, suggesting a lack of functionality of the septation initiation network (SIN, the MEN homolog in this organism), whose components also localize to the SPBs and initiate a signal that emanates from these structures to trigger cell separation [Citation10].
The previous examples illustrate the relevance of the localization of cell cycle regulators on the SPBs during the final stages of mitosis. However, many other cell cycle events from mitotic entry to cytokinesis are also controlled by signals that emanate from the SPBs. To regulate these processes, proteins such as Cdk1 (the only member of the CDK family in budding yeast), as well as other key kinases and phosphatases that act as global regulators of mitosis, also localize on the SPBs at some point to coordinate the timely progression throughout the cell cycle. These MTOC-regulated cellular events include the duplication of the SPBs themselves and the precise control of their number within the cell. In this way, Cdk1, the POLO-like kinase Cdc5 and Cdc14 work together on the SPBs to ensure that duplication of these structures takes place only once every cell cycle in S. cerevisiae [Citation11]. On the other hand, SPB-localized Swe1 kinase specifies the identity and fate of these MTOCs during their segregation by phosphorylating the MEN-component and SPB structural constituent Nud1 protein [Citation12]. Finally, MEN signaling on the SPBs and recruitment of Cdc14 to these structures also play an important role in septin ring splitting and actomyosin ring constriction during cytokinesis [Citation13]. The role of Cdc14 in the regulation of cytokinesis is proposed to be, in fact, the most conserved function of this family of phosphatases throughout evolution [Citation14].
Localization of essential regulatory proteins to the SPBs is also fundamental during cell division by meiosis. As such, Ipl1, the only member of the Aurora kinase family in budding yeast, is required to maintain SPB cohesion during meiosis I, while Cdc5 kinase is later required to inhibit its role and promote SPB separation [Citation15]. On the other hand, Cdk1 has been found to oppose a role of Ipl1 in preventing spindle formation during the meiotic divisions [Citation16]. One last example of the pivotal role of SPBs during meiosis is the regulation of the activity of a yeast development-specific protein phosphatase 1 (PP1) complex by means of the dynamic control of its association with these structures. Remarkably, and while this PP1 complex initially associates with the SPBs as the prospore membrane forms, its release from these MTOCs is later necessary for prospore membrane extension during sporulation [Citation17].
Similar to SPBs, centrosomes are also the center for the transmission of intracellular signals that coordinate cell cycle progression in higher eukaryotes. Accordingly, cells arrest in G1 when centrosomes are eliminated either by microsurgery or using laser ablation [Citation18,Citation19]. As observed in yeast, essential cell cycle regulators have been shown to associate to centrosomes in cells from Drosophila melanogaster, Caenorhabditis elegans, mice and many other organisms, including humans, where these proteins regulate and coordinate multiple events in every phase of the cell cycle. In this way, cyclin E associates with centrosomes from G1 through S phase in Chinese hamster ovary (CHO) cells [Citation20]. Furthermore, a 20 aa domain within this protein was identified as a specific centrosomal localization signal (CLS) that is required for targeting to the spindle MTOCs [Citation20]. A similar CLS motif was later found for cyclin A, which also localizes to these structures in CHO, Xenopus S3 and human HeLa cells [Citation21]. The localization of both cyclin A and E to centrosomes is thought to be important for targeting and recognition of CDK substrates at this location, thereby spatially regulating their activities in order to promote key cell cycle events. Accordingly, truncation of the CLS in either cyclin E or cyclin A interferes with S phase entry and reduces the fraction of cells carrying out DNA synthesis [Citation20,Citation21]. Excitingly, these observations establish an interesting connection between MTOCs and the control of DNA replication. Not only cyclins E and A, but also cyclin B associates to centrosomes [Citation22]. Interestingly, localization on the spindle MTOCs facilitates cyclin B retention on these structures, thereby preventing it from fulfilling its mitotic activity within the nucleus until its activation by cytoplasmic Cdc25B [Citation22]. Further supporting the role of MTOCs as central signaling platforms, the centrosomal kinase activity of another key cell cycle regulator, Aurora A, also seems to be important for the initial activation of cyclin B1-Cdk1 at this location [Citation23]. In contrast with cyclins E and A, the regulation of cyclin B1 at the centrosomes is not limited to its activity on these structures, being instead responsible for generating a local inhibitory signal that extends to the cytoplasmic pool of cyclin B1-Cdk1 complexes. This observation highlights the versatility of the MTOCs as signaling centers where the activity of pivotal cell cycle regulators, and therefore the progression through the different mitotic transitions, can be modulated in multiple ways, affecting a variety of essential processes that take place not only within these structures but throughout the cell.
Proteins from the Polo family are additional examples of evolutionary conserved cell cycle regulators that, as Cdc5, also display centrosomal localization in higher eukaryotes. In mammalian cells, different members of this family, such as the human Plk1 and Plk4 kinases [Citation24,Citation25], associate with these structures. Localization of Plk1 is highly dynamic, and it starts loading on centrosomes from G2 to prophase [Citation26,Citation27]. Remarkably, the initial activation of Plk1 activity during the cell cycle occurs precisely at this stage, and it is thought to take place once the kinase is at the centrosomes. Next, as cells enters prometaphase, Plk1 gets also enriched in kinetochores, to finally gradually disappear from centrosomes after chromosomes segregate during anaphase [Citation26,Citation27]. As for cyclin B1-Cdk1, initial centrosomal activation of Plk1 is mediated by Aurora A [Citation28,Citation29]. Plk1 activity is then further enhanced by Bora, an Aurora A cofactor, to promote full activation of the Polo kinase in the nucleus [Citation30]. At the centrosomes, Plk1 regulates the maturation, disjunction, separation and number of these structures [Citation26], as well as their function in microtubule nucleation [Citation31]. Interestingly, Plk1 promotes centrosome disjunction partly by activating the Hippo pathway, the mammalian homolog of the S. cerevisiae MEN signaling cascade [Citation32]. Centrosomal Plk1 further controls mitotic spindle positioning, as demonstrated in human HeLa cells [Citation33]. Another member of the Polo family, Plk4, also localizes to the spindle MTOCs, where it plays important roles in centriole duplication, the stimulation of the microtubule-nucleating activity of centrosomes and spindle assembly [Citation34–Citation37]. In this way, Plk4 facilitates centriole duplication by engaging important factors such as STIL or Sas-6 [Citation34,Citation35]. The Plk4-mediated phosphorylation of STIL facilitates recruitment of Sas-6 to the centrioles, thus favoring the initiation of the pro-centriole assembly process [Citation34,Citation35]. Furthermore, the concerted action of Plk4 and its associated factors prevent the formation of extra centrioles to ensure the maintenance of the correct number of these structures in the cell [Citation35,Citation38]. Plk4 auto-phosphorylation promotes its destruction by the proteasome, which establishes a self-regulatory pattern of regulation of its activity that safeguards proper centriole duplication [Citation39,Citation40]. Remarkably, this serine/threonine protein kinase can even promote de novo centriole formation in cells lacking these structures, based on its capacity to self-organize into condensates that recruit centrosomal components and α/β- and γ-tubulin to give rise to functional MTOCs that have the ability to nucleate microtubules [Citation41]. Therefore, Plk4 not only carries out important regulatory functions at the MTOCs, but it also plays an essential structural role during the biogenesis of these structures [Citation41].
The homologs of the PP1, PP2A (protein phosphatase 2A) and Cdc14 phosphatases also display centrosomal localization in higher eukaryotes. These proteins act in many cases by counteracting the activity of the aforementioned kinases. As such, PP2A seems to play an important role promoting centrosome duplication during mitosis in Drosophila embryos by inhibiting the auto-phosphorylation of Plk4, thereby preventing its degradation by the proteasome and favoring the accumulation of this kinase on the centrosomes [Citation42]. The role of PP2A in centrosome duplication seems to be conserved. However, in contrast to what found in Drosophila, PP2A was proposed to act downstream of ZIG-1 (the Plk4 homolog) in C. elegans embryos. Specifically, PP2A dephosphorylates Sas-5, an essential factor required for centriole formation that forms a complex with Sas-6, this way promoting centrosomal localization of the Sas-5/Sas-6 complex in the worm embryo [Citation42,Citation43]. PP2A also protects ZIG-1 and Sas-5 from degradation, regulating their protein levels [Citation42]. Strikingly, PP2A has also been shown in C. elegans embryos to later further oppose Plk1 and Aurora A function on the centrosomes to promote the disassembly of the pericentriolar material (PCM) as cells exit mitosis, thereby decreasing microtubule nucleation [Citation44]. Finally, and besides these functions in centrosome duplication, the PP2A phosphatase, in a heterotrimeric complex with its associated B55/SUR-6 adaptor subunits, was found to act in collaboration with the nuclear lamina to promote centrosome separation during mitotic entry [Citation45]. Therefore, PP2A activity on the spindle MTOCs must be precisely and differentially regulated to act on diverse substrates and thus to carry out unique functions at different cell cycle stages. Among other strategies, this can be achieved by the presence of multiple isoforms of PP2A that result from the combination of a variety of structural, catalytic and regulatory subunits [Citation46].
Interestingly, and despite centrosomal localization of PP1βGSP−1 could not be observed in C. elegans embryos, ZIG-1 levels have been also shown to depend on this isoform of the PP1 phosphatase [Citation47]. Reduction of PP1 activity, either directly or indirectly, leads to an increase both in the overall ZYG-1 levels and in the localization of this Polo kinase on centrioles during prophase and metaphase, and also determines an amplification in the number of centrioles. This indicates that both ZYG-1 and PP1 participate in a common regulatory pathway that is important in order to restrict centrosome number in worm embryos [Citation47]. Furthermore, a direct link between centrosome-localized PP1 and the Plk1/Aurora A regulatory module has been proposed in human U2OS cells. Specifically, CEP192, a protein that plays an essential role in the localization of Aurora A to centrosomes [Citation48], was shown to also help recruiting PP1 to these structures via a characteristic RVxF motif [Citation49]. Interestingly, this interaction with PP1 is inhibited by the Plk1-mediated phosphorylation of the RVxF motif in CEP192 and was suggested to be important for the correct control of CEP192 function on the centrosomes, which is required for proper centrosome size determination, microtubule nucleation and bipolar spindle assembly [Citation48,Citation49]. An additional role of PP1 has been proposed in the BRCA1-mediated regulation of centrosome maturation and microtubule nucleation. Specifically, PP1α-mediated BRCA1 dephosphorylation during interphase would increase its ubiquitin ligase activity, which promotes targeting of γ-tubulin for its proteasome-dependent degradation and the subsequent inhibition of centrosome maturation [Citation50]. This inhibitory function of PP1α and BRCA1 would later be antagonized by AURKA during mitotic entry [Citation50]. Finally, PP1 has also been shown to collaborate with Plk1 and the Hippo pathway in the regulation of the Nek2 kinase on the centrosomes, whose activity promotes the separation of these structures [Citation32,Citation51].
Phosphatases from the Cdc14 family localize on centrosomes in C. elegans, Xenopus, zebrafish, chicken, mouse and human cells [Citation52–Citation57]. Interestingly, two different isoforms of the Cdc14 phosphatase, hCdc14A and hCdc14b, are expressed in human cells, which display distinctive localizations. While hCdc14A is excluded from the nucleoli and localizes on the centrosomes from interphase to G2-M transition, hCdc14B is observed in nucleoli [Citation55]. This separate compartmentalization of both hCdc14 isoforms suggests that the functions carried out by the single Cdc14 protein in budding yeast could be differentially distributed between hCdc14A and hCdc14B in human cells. Remarkably, and in agreement with a role of hCdc14A in maintaining the correct centrosome number, increased expression of hCdc14A determines premature centrosome disjunction in U2OS human cells while, conversely, its inactivation leads to defects in centrosome duplication [Citation55]. In Xenopus embryos, an initial study identified two different paralogues of hCdc14A, named XCdc14α and XCdc14β, but no evident hCdc14B homolog [Citation58]. It was later found, however, that XCdc14β is encoded by a gene that is subjected to alternative splicing, also giving rise to a protein closely related to hCdc14B, which was consequently named as XCdc14B [Citation59]. Remarkably, as described for hCdc14A, XCdc14A was found to localize to centrioles during interphase in both Xenopus cells and a heterologous human HeLa cells system [Citation59]. Overexpression of XCdc14A in the heterologous HeLa model impaired microtubule nucleation from centrosomes, suggesting a role of the Xenopus paralogue in centrosomal function [Citation59]. Furthermore, ectopic XCdc14A expression interferes with both mitotic entry and cytokinesis, supporting a role for this protein in cell cycle control [Citation58,Citation59]. Regulation of mitotic entry by XCdc14A was proposed to be mediated through dephosphorylation of the CDK activator Cdc25. On the other hand, cells displaying the cytokinesis defects showed a similar phenotype to that of centriolin mutants, which cannot recruit essential abscission factors to the midbody and thus cannot separate cells, giving rise to interconnected daughter cells that are unable to progress into the next S phase [Citation59]. Remarkably, centriolin is the homologue of the SPB component Nud1 [Citation60], the anchor for MEN proteins on these structures in S. cerevisiae, highlighting once again that similar strategies are used to regulate essential processes at the spindle MTOCs in cells from different eukaryotic organisms.
The recurrent localization of proteins that belong to the same families of key cell cycle regulators to the spindle-associated MTOCs from yeast to human cells demonstrate the evolutionary conservation of the role played by these structures as central regulatory hubs where cell cycle signaling pathways converge and are coordinated. Likewise, the multiple interconnections showed by all these fundamental cell cycle kinases and phosphatases during the control of diverse aspects of the SPB/centrosome cycle and the regulation of the cell cycle highlights the complexity of this MTOC-centered signaling network and the relevance of the SPBs and centrosomes beyond their function in microtubule nucleation.
Checkpoint signaling from spindle-associated MTOCs
To ensure the fidelity of chromosome replication and distribution, different surveillance mechanisms allow cells, once triggered, to temporarily halt the progression of mitosis or meiosis in order to provide them with the time to solve and repair any problem that could arise. Remarkably, the MTOCs that nucleate the spindle also constitute a central regulatory center to coordinate these checkpoint responses. During cell division, the integrity of the genome is monitored by the DNA damage checkpoint (DDC), which is activated by a number of highly conserved proteins that sense lesions in the DNA and trigger a cellular response that includes different mediators and effector proteins and both restrain cell cycle progression and promote DNA repair [Citation61,Citation62]. Among the initial components that are activated after the DDC is triggered are the Ataxia-telangiectasia mutated (Tel1 in S. cerevisiae/ATM in mammals) and the ATM and Rad3-related (Mec1/ATR) protein kinases [Citation61,Citation62]. In turn, activation of Mec1/ATR and Tel1/ATM triggers a complex signaling cascade that eventually promotes the activity of effector kinases such as Chk1/CHK1 and Rad53/CHK2, which establish two separate branches from the DDC [Citation61,Citation62]. Among the targets of these kinases, the Cdc14 phosphatase has been shown to play an essential role in the response to DNA damage in S. cerevisiae. Interestingly, Cdc14 is not only released from the nucleolus at anaphase onset. After induction of a DNA double-strand break (DSB), a small fraction of this phosphatase is released into the nucleoplasm and then targeted to the SPBs, where it is necessary to recruit DSBs to these structures, thereby enhancing DNA repair by homologous recombination [Citation63]. Additionally, loading of Cdc14 to the SPBs is necessary in order to stabilize and correctly orient the metaphase spindle during the DDC-dependent cell cycle arrest [Citation63]. Remarkably, maintenance of this DNA damage-induced block in mitotic progression further requires the inhibition of mitotic exit mediated by the Bfa1/Bub2 complex, a two-component GTPase-activating protein (GAP) that also localizes to the SPBs [Citation64]. Bfa1/Bub2 are MEN components that are necessary for Tem1 loading onto the SPBs and negatively regulate the activity of this GTPase until anaphase. Once in anaphase, Cdc5 phosphorylates and inhibits Bfa1/Bub2, thus promoting Tem1 activation and MEN signaling [Citation64–Citation66]. However, when the DDC is triggered, Rad53 inhibits Cdc5, which prevents Bfa1/Bub2 inactivation by the Polo-like kinase and restrains mitotic exit [Citation67]. Furthermore, Rad53 might also contribute to Bfa1/Bub2 inhibition by interfering with Cdc5 localization to the SPBs [Citation67]. Intriguingly, the Rad53-dependent Cdc5 inhibition, despite being generally observed after DDC activation, it is specifically required to maintain the DDC-induced cell cycle arrest in response to DNA damage to the telomeres [Citation67].
Exit from mitosis is a particularly important cell cycle transition that needs to be strictly regulated and blocked when genome integrity or distribution is compromised in budding yeast, as evidenced by the fact that its inhibition is promoted by the main mitotic checkpoints and required to maintain their functionality, despite these surveillance mechanisms being triggered by diverse signals and at different stages of the cell cycle [Citation5,Citation66–Citation69]. Remarkably, the Polo-like kinase Cdc5 seems to be a central target of these checkpoints to preclude mitotic exit. In this way, not only the DDC, but other critical surveillance mechanisms acting later in the cell cycle such as the spindle-assembly checkpoint (SAC), which is activated by the lack of attachment of the chromosomes to the spindle, or the spindle orientation checkpoint (SPOC), which ensures that cells do not exit mitosis until the spindle is correctly positioned, also rely on the ability of Cdc5 to phosphorylate Bfa1/Bub2 and thus to maintain Tem1 in an inactive state and inhibit MEN signaling [Citation66,Citation68,Citation69].
The SPOC is a particularly interesting checkpoint, since it probably constitutes the best example of the importance of the MTOCs in checkpoint signaling. Moreover, this surveillance mechanism is also an outstanding paradigm of how the asymmetric distribution of key regulators to these structures can be exploited in order to convey spatial information to ensure the correct coordination between genome distribution and cell cycle progression. Interestingly, Tem1, together with Bfa1/Bub2, asymmetrically localize to the SPB that enters the daughter cell during anaphase, while they are excluded from the SPB that remains in the mother cell () [Citation64,Citation70,Citation71]. The differential distribution of these proteins does not depend on SPB age, but on the interactions that the microtubules that emanate from these structures establish with the daughter cell cortex [Citation71]. The main SPOC effector, the Kin4 kinase, is also asymmetrically distributed and localizes to the mother cell cortex throughout the cell cycle [Citation68,Citation69]. A fraction of the Kin4 protein also transiently binds to the SPBs at the metaphase-to-anaphase transition (). Remarkably, however, and in contrast with Tem1 and Bfa1/Bub2, Kin4 exclusively loads on the SPB that remains within the mother cell, where it prevents the inhibitory phosphorylation of Bfa1/Bub2 by Cdc5, this way precluding Tem1 activation [Citation68,Citation69]. Additionally, once on the SPBs, Kin4 also destabilizes binding of Bfa1/Bub2 to these structures [Citation71,Citation72]. Since Tem1 loading on the SPBs is dependent on Bfa1/Bub2, this further avoids Tem1 localization to these MTOCs, which is a requirement for MEN signaling, thus potentiating the inhibition of mitotic exit [Citation5]. The exclusion of Kin4 from the daughter cell SPB is facilitated by Lte1, a MEN activator that asymmetrically localizes to the bud cortex and promotes Tem1 activity during anaphase () [Citation73,Citation74]. By means of the differential distribution of these proteins, budding yeast cells manage to exquisitely coordinate spindle position with mitotic progression and ensure a correct distribution of the duplicated genome. The localization of Kin4 and Lte1 to the mother and daughter cell cortex, respectively, establishes MEN-inhibitory (the mother cell) and MEN-promoting (the bud) cellular territories, so that only when the spindle is correctly oriented and the SPB that bears Tem1 enters the daughter cell after spindle elongation, the activity of the GAP is promoted and mitotic exit can take place (). However, when the spindle is not aligned along the polarity axis and elongate within the mother cell, Kin4 loads on both SPBs and strongly inhibits MEN signaling by simultaneously stimulating the Bfa1/Bub2-dependent inhibition of Tem1 activity and promoting the exclusion of Bfa1/Bub2, and hence of Tem1, from the SPBs [Citation75].
A puzzling observation is that, after a prolonged cell cycle arrest promoted by the activation of the previous surveillance mechanisms, cells can bypass the checkpoint-induced block and resume mitotic progression despite the problem that originally triggered the response still persisting. This process is known as checkpoint adaptation, and it is thought to be a way to ensure the survival of at least a fraction of the cells in a population, in contrast to a permanent arrest that would imply the total extinction of the population, notwithstanding the magnitude of the problem that triggered checkpoint activation [Citation76]. The process of checkpoint adaptation was originally observed in budding yeast. Interestingly, in this organism, Cdc5 also plays an essential role in the adaptation to the checkpoint and the subsequent bypass of the cell cycle block [Citation77]. Despite the initial activation of Rad53 by the DDC leading to Cdc5 nuclearization, an extended G2/M arrest caused by an irreparable DSB eventually drives a re-localization of the Polo-like kinase, which gets enriched at the SPBs [Citation67,Citation78]. Remarkably, this targeting of active Cdc5 to the SPBs is required to mediate the adaptation to persistent DNA damage, highlighting again the crucial role of the MTOCs in the regulation of the functionality of the mitotic checkpoints [Citation79]. Moreover, the adaptation defect of cdc5 mutants defective in the phosphopeptide-binding activity of the Polo-binding domain (PBD), which cannot localize to the SPBs, can be recovered both by artificially tethering a Cdc5 mutant protein lacking the PBD to these structures and by deleting the BFA1 gene. This last observation demonstrates that checkpoint adaptation to persistent DNA damage is counteracted by Bfa1/Bub2 activity on the SPBs and that a pivotal function of Cdc5 in this process is to prevent this negative regulation of the checkpoint bypass mediated by the Tem1 GAP [Citation79].
As in budding yeast, centrosomal localization of key checkpoint regulators is essential for the functionality of these surveillance mechanisms in higher eukaryotes. Interestingly, the DDC component Chk1 regulates the centrosomal localization of cyclin B1 already during a normal cell cycle in U2OS cells [Citation22]. Centrosome-associated cyclin B1-Cdk1 is initially maintained in an inhibited state by Chk1 as these cells enter and progress through the cell cycle, which prevents unscheduled activation of this complex. Then, once in prophase, and concomitant with separation of the duplicated MTOCs, Chk1 disappears from the centrosomes and cyclin B1-Cdk1 can be activated at this location by cytoplasmic Cdc25B [Citation22]. This Chk1 function contributes to maintain the accurate timing of cyclin B1-Cdk1-dependent processes such as mitotic spindle formation [Citation22]. Remarkably, however, after treatment with various DNA-damaging agents, Chk1 mediates a cellular response that induces centrosome amplification by promoting an excessive formation of centriolar satellites that act as assembly platforms for centrosomal proteins [Citation80]. This results in an excessive number of centrosomes, which determine chromosomal instability and the elimination of the cells due to mitotic catastrophe and the subsequent arrest in G0 or cell death () [Citation80,Citation81]. The induction of mitotic catastrophe associated to centrosomal defects might be in fact a conserved response to DNA damage [Citation81]. As such, evidences in Drosophila embryos and mammalian cells show that DNA lesions can lead to centrosome inactivation or fragmentation, which also cause chromosomal instability and triggers mitotic catastrophe [Citation82,Citation83]. Remarkably, in Drosophila embryos, this response induces recruitment of the DDC effector Chk2 (DmChk2 in flies) to the centrosomes, which is required to their inactivation [Citation82].
Polo kinases also exemplify again the strong conservation of the mechanisms regulating spindle MTOC-associated cellular checkpoint responses. Protein from the Polo kinase family are fundamental targets of the pathways that are triggered after the induction of the main mitotic checkpoints. Indeed, activation of the DDC after DNA damage leads to a strong inhibition of Plk1 activity that takes place at different levels [Citation84,Citation85]. In this way, not only Plk1 activity is directly inhibited and its degradation also promoted once that the DDC is triggered, but upstream activators of Plk1 like Aurora A or Bora are additionally repressed. In the presence of DNA damage, Chk1 induces the inactivation of Aurora A, while ATR targets Bora for degradation and Plk1 binding to Bora is further prevented by the DDC-mediated inhibition of Cdk [Citation85–Citation89]. Remarkably, during the initial stages of the response to DNA damage, Plk1 activity is inhibited by blocking Aurora A recruitment to the Plk1/Bora complex [Citation85]. Hence, it is possible that this initial inhibitory step could take place at the centrosomes and might be mediated by Chk1 or a different DDC component localized to these structures. Shockingly, despite Plk1 being repressed by the DDC, the activity of this kinase is later required for repair of the damage, as suggested by the fact that phosphorylation by Plk1 is required for Rad51 recruitment to the DNA lesions, thereby promoting repair by homologous recombination [Citation90].
PP1 illustrates a final example of another key transducer of centrosome-associated checkpoint signals in higher eukaryotes. Ionizing radiation causes the activation of PP1α, a checkpoint-specific isoform of PP1 that regulates centrosome separation and number in an ATM-dependent manner [Citation91]. PP1α preferentially localizes to the centrosomes and, once active, binds to and dephosphorylates the NIMA-like kinase Nek2, thereby inhibiting its function, which is required to initiate centrosome splitting and helps maintaining genome stability [Citation91]. Remarkably, Nek2 is additionally regulated in an ATM-dependent manner by Plk1. The inhibition of Plk1 promoted after DNA damage prevents Nek2 phosphorylation by Polo, thereby inhibiting its activity and further blocking centrosome separation [Citation92]. This regulation exemplifies the complexity and the variety of the signals that are integrated at the level of the centrosomes to coordinate the cellular response to DNA lesions.
A link between MTOCs and cell metabolism
Cell division is a highly energy-demanding process and thus cell cycle progression and nutrient availability must be tightly coordinated. To this end, a two-way crosstalk ensures not only the rewiring of the main metabolic pathways throughout the different phases of cell division by mitosis but also that entry and progression through the cell cycle only take place under optimal nutritional conditions. Remarkably, despite the precise molecular mechanisms linking the cell metabolic state with the control of the cell cycle are far from being completely understood, the most important nutrient sensing pathways display connections to effectors and regulators of mitosis such as cyclin-CDK complexes, some of which associate to the spindle MTOCs. In this way, the target of rapamycin (TOR) pathway, a central network that modulates cell growth in response to nutrient availability from yeast to mammals, controls the G2/M transition in S. pombe by promoting the recruitment of the Polo kinase Plo1 to the SPBs and hence facilitating the subsequent activation of the mitotic onset regulator Cdc2 [Citation93]. Similarly, inactivation of the TORC1-PP2A phosphatase pathway severely affects the nucleo-cytoplasmic transport and SPB localization of Cdc5 and the stability of cyclin CLB2 mRNA in S. cerevisiae [Citation94,Citation95]. Interestingly, checkpoint signaling seems to also be interconnected with nutritional signals. As such, the cyclic-AMP-dependent protein kinase (PKA) pathway, which responds to carbon source availability, and the DDC converge on APC/C-Cdc20 substrates to regulate mitotic progression [Citation96]. Similarly, the morphogenesis checkpoint Swe1 kinase, which localizes to the SPBs [Citation12], is activated in lipolysis-defective conditions and halts cell cycle progression through Cdc28 phosphorylation at the G1/S transition [Citation97]. Despite no direct connection with MTOCs has been so far established, the pivotal function of these structures as central regulatory hubs for checkpoint signaling makes it feasible for MTOC-associated proteins to play an important role in the interaction between these surveillance mechanisms and nutrient sensing pathways.
Fascinatingly, a growing list of proteins that belong to metabolic networks is emerging that physically associate to the mitotic apparatus and, specifically, with the spindle MTOCs [Citation98]. In this way, a phospho-active subunit of the AMP-dependent kinase (AMPK) is rapidly relocated to centrosomes at the beginning of mitosis, where it is phosphorylated by Plk1 to then travel to the spindle midzone () [Citation99,Citation100]. At this location, AMPK has been proposed to regulate anaphase spindle length by phosphorylating the KIF4 kinesin [Citation101]. Similarly, Mio, a conserved member of the SEACAT/GATOR2 complex that is necessary for mTORC1 activation, plays a critical role in the stimulation of Aurora A and Plk1 kinase activities at the centrosomes [Citation102]. Not only mTOR pathway elements, but proteins of other nutrient signaling networks can be also observed at MTOCs. Indeed, PKA pathway components also localize to centrosomes in HeLa cells during prophase and prometaphase and inhibition of PKA activity in these cells induces mitotic defects that can result in aneuploidies [Citation103]. However, whether the function of these proteins in regulating mitotic progression is directly connected to their role in nutrient and energy-sensing is an open question that needs to be elucidated in the future. Interestingly, a series of observations additionally suggests that metabolic enzymes could localize to centrosomes or interact with some of their components specially in the context of tumor cells, although again the connection with cell metabolism remains unclear in most cases. In this way, human asparagine synthetase (ASNS), an enzyme that is overexpressed in many cancer types, has been shown to colocalize with the centrosomal marker Aurora A just prior mitotic entry [Citation104]. Yet, whether ASNS could play a role in spindle assembly/function or, alternatively, whether it might be recruited to the spindles and centrosomes to provide a localized source of asparagine is also an unsolved conundrum [Citation104].
Signaling from the spindle MTOCs seems to not only integrate signals that regulate cell metabolism but to also respond to oxygen levels. Hypoxia is long known to modulate mitotic progression and can induce a G1/S cell cycle arrest [Citation105,Citation106]. Human prolyl-4-hydrolases PHD1–3 are important in the cellular response to oxygen levels. The activities of PHDs decrease upon hypoxia, leading to a stabilization of hypoxia-inducible transcription factors (HIFs) [Citation107]. Remarkably, PHD1 plays an essential role in centrosome duplication and maturation through modification of the centrosome component Cep192, targeting it for proteasomal degradation and thus modulating the levels of this protein and contributing to the regulation of cell cycle progression [Citation108,Citation109].
Although the link between MTOCs, cell metabolism and energy sensing is still widely unexplored and the connection between metabolic enzymes and MTOC-regulated cell cycle-dependent processes in many cases respond to moonlighting activities of these proteins [Citation104,Citation109], the abovementioned observations suggest that SPBs and centrosomes also act as regulatory centers that coordinate signaling from metabolic and energetic pathways with the control of cell cycle progression and the functionality of the mitotic checkpoints.
Intracellular localization and distribution of cellular components through their association to MTOCs
The main function that has been historically associated with the SPBs and the centrosomes, despite it is now evident that spindle-associated MTOCs constitute essential regulatory nucleuses where multiple signaling pathways converge, is the nucleation of the microtubules that build the mitotic spindle and facilitates the even partition of the replicated genome during cell division. The differential attachment of each of the sister chromatids of every chromosome to microtubules that emanate from the daughter-directed or the mother-retained SPB facilitates their equal distribution during mitosis [Citation3]. Similarly, the separation of homolog chromosomes during meiosis I and the subsequent distribution of sister chromatids during meiosis II that characterizes the stereotypical reductional pattern of chromosome segregation during meiosis is also mediated by an exquisitely regulated association scheme of the DNA molecules with the microtubules that radiate from the spindle MTOCs [Citation110]. Remarkably, the segregation of these MTOCs during cell division is further exploited in order to deliver many other different cellular components. Molecules other than DNA are distributed by association with the spindle MTOCs between the dividing cells, a partition that can be symmetric or asymmetric. In this way, the preferential allocation of proteins to the mother or the daughter cell constitutes an outstanding strategy to differentially regulate their expression and/or activity in both compartments, as exemplified by the importance of the asymmetric localization Bfa1/Bub2, Tem1 or Kin4 for the proper coordination of spindle position with mitotic exit [Citation64,Citation68–Citation71]. Fascinatingly, such an elaborate mechanism for the modulation of the spatial regulation of gene expression has also been postulated to take place at the mRNA level (). A number of seminal studies suggested an association of a fraction of the mRNA pool in animal cells with the mitotic apparatus and the cytoskeleton [Citation111,Citation112]. However, it has not been until recently that evidences supporting a direct functional link between RNA molecules and the centrosomes started to accumulate. One first indication pointing toward this association was described in the dividing Xenopus embryo, where the mRNA coding for cyclin B1 was shown to concentrate on the mitotic spindle and centrosomes during development [Citation113]. This observation further substantiates the essential role of spindle MTOCs in the regulation of cell cycle progression, as it might indicate that the specific localization of the cyclin B1-encoding mRNA to centrosomes could help to locally express this regulatory CDK subunit and to promote its accumulation on these structures, thus enhancing its kinase activity at this location or facilitating its transport coupled to MTOC-distribution during cell division. Later studies in mollusks support the idea that mRNAs are indeed transported to the spindle MTOCs in a microtubule-dependent manner and that they could be distributed by means of their association with these structures. As such, the transcripts of a number of developmental genes RNAs, including those for Even-skipped (Eve), Decapentaplegic, and Tolloid genes, associate with centrosomes in Ilyanassa obsoleta embryos, which facilitates their asymmetric distribution during cell division and the generation of elaborated patterns of gene expression based on the differential localization of the different mRNA molecules [Citation114]. Remarkably, in fact, up to 3–4% of all the RNA in the embryo of I. obsoleta concentrate at some point at the centrosomes during early development [Citation115]. The existence of centrosome-localized RNA molecules in other organisms, such as in the fruit fly D. melanogaster [Citation116], have been also confirmed afterward, further supporting the previously described initial evidences in Xenopus and mollusks.
Cellular organelles also associate with the spindle MTOCs for their segregation. As such, the connection of part of the mitochondria with the SPBs seems to play an important role for their distribution during mitosis in fission yeast [Citation117]. Similarly, the analysis of mdm1 mutants in S. cerevisiae suggested the existence of a common scaffold-like protein network that facilitates mitochondrial inheritance and spindle positioning. Based on this role of Mdm1 in both nuclear and mitochondrial inheritance, a speculative model opening the door to a simultaneous tethering of SPBs and mitochondria to the Mdm1-associated network was also proposed in budding yeast [Citation118]. Finally, a potential link between MTOCs and mitochondria has been also suggested in other organisms including humans [Citation119–Citation125].
As for mitochondria, an exciting nexus between the Golgi apparatus and the spindle MTOCs has been also observed and thoroughly analyzed during the years. However, their relationship is still far from being completely understood and whether their association has a functional meaning is still a matter of debate [Citation126,Citation127]. Cumulative evidence points toward a bidirectional relationship between both organelles, with the Golgi apparatus being important for proper centrosome integrity, allocation and function while, conversely, microtubules that emanate from the MTOCs playing a fundamental role in the localization of the Golgi in the physical proximity of these structures, which could contribute to maintain polarized vesicular trafficking [Citation126,Citation128,Citation129]. In contrast to this view, however, recent studies in RPE-1 human cells seem to suggest that the Golgi-centrosome proximity is dispensable for directional protein transport, directorial migration, ciliogenesis and mitotic entry [Citation127]. Further research effort will be therefore necessary in the future in order to clarify the functional meaning of the Golgi-centrosome association.
Endosome-associated proteins also display centrosomal localization. In human cells, endosomal components such as Sec15, Sec6, Rab11 and its GTPase Evi5 associate with MTOCs [Citation130]. The demonstration of a direct interaction between Rab11 and the centrosome appendage protein cenexin further suggested a structural and functional link between endosome and centrosome [Citation130]. Based on these evidences, a hierarchy in the organization of centrosome and endosome proteins has been proposed in human cells that facilitates the association of the recycling endosome with the mother centriole. Specifically, cenexin anchors centriolin, another centriolar protein, which subsequently allows the association of Sec15 and Evi5 with the mother centriole. Cenexin further facilitates the association of GTP-bound Rab11 and the exocyst with the centrosome. Finally, and once on the centrosome, Evi5 would control the activity of cenexin-bound Rab11 at the mother centriole, thereby regulating endocytic recycling [Citation130]. Remarkably, and similarly to what postulated for the Golgi, it has been suggested that the relationship between the endosome and the centrosomes is likely to be bidirectional, so that the function of the MTOCs could be itself regulated through their association with the endosome too [Citation130]. Interestingly, in Drosophila embryos, the Rab11 effector Nuf also displays a cell cycle-dependent centrosomal localization that is regulated by changes in its phosphorylation state mediated by Cdk1 and Polo [Citation131], once again highlighting the pivotal role of these proteins in the regulation of multiple processes that turn spindle MTOCs into central signaling hubs in the cell.
Asymmetric inheritance of SPBs and cellular aging
After their duplication during the initial stages of the cell cycle, the preexistent (“old”) and the newly-generated (“new”) MTOCs are intrinsically different both in morphology and in age [Citation132]. Fascinatingly, germinal studies in S. cerevisiae demonstrated that in this organism the duplicated SPBs are asymmetrically distributed during each cell division, so that the old SPB always segregates toward the daughter cell while the new SPB is retained by the mother [Citation133]. A predetermined pattern of spindle MTOC inheritance was later found to be displayed by many cells from different organisms that also divide asymmetrically, suggesting that it is likely an evolutionary conserved phenomenon. In this way, the self-renewing Drosophila male germline stem cell (GSC) retains the older centrosome during its asymmetric division, while the newly-generated MTOC is inherited by the differentiating daughter cell [Citation134]. Interestingly, however, the pattern does not necessarily need to always be the same. In fact, an opposite preferential pattern of MTOC distribution has been described for the female GSCs and the neuroblasts of the same organism, which maintain the newer centrosome and segregate the older MTOC to the differentiating cell that arises after the asymmetric division of the stem cell [Citation135,Citation136]. Predetermined patterns of centrosome distribution have also been described in stem cells from the mouse neural cortex and human neuroblastoma cells [Citation137,Citation138]. Remarkably, despite cumulative evidences supporting the conservation of this phenomenon as well as the new insights provided into the molecular mechanisms guiding the differential distribution of SPBs and centrosomes during asymmetric cell divisions, the functional meaning of this process has long been a subject of debate. Recent work from our laboratory has shed new light on this intriguing question. By generating a S. cerevisiae strain that displays a constitutively reversed SPB inheritance pattern, we have demonstrated that the differential fate of these structures during mitosis is essential in order to maintain the replicative lifespan of the cells [Citation139]. In budding yeast, the mother cell continuously ages as it goes through each consecutive division, but the newly-generated daughter cell is rejuvenated and maintains its full lifespan. This rejuvenation of the daughter cells is enabled by the specific retention of different damaged molecules and organelles in the mother cell, such as extrachromosomal rDNA circles (ERCs), carbonylated and aggregated proteins, fragmented and oxidized mitochondria, etc [Citation140]. Our results indicate that segregation of the old SPB into the daughter cell is critical to maintain correct levels of the Sir2 sirtuin (a histone deacetylase that has been long associated with aging) and proper distribution of the mitochondrial inheritance regulator Mfb1, and therefore to ensure a proper distribution of functional mitochondria and protein aggregates during the asymmetric division of S. cerevisiae () [Citation139]. The alteration of these processes as a consequence of the forced retention of the old SPB into the mother cell accelerates cellular aging, uncovering for the first time a biological role for the asymmetric distribution of spindle MTOCs during cell division [Citation139]. Budding yeast have been extensively used as a model organism to analyze asymmetric cell divisions and replicative aging in stem cells [Citation140]. As stem cells age they also show symptoms of progressive functional decline. Excitingly, and in agreement with the observations in budding yeast, Drosophila male GSCs that cannot properly orient the centrosomes during their division produce a drop in spermatogenesis during aging in the fly [Citation141]. Furthermore, our results in S. cerevisiae agree with the opposite patterns of centrosome inheritance described for Drosophila male GSCs and neuroblasts. Remarkably, in Drosophila testis the GSC outlives the gonial cell that is generated after its asymmetric division, being the stem cell that receives the old centrosome [Citation134]. However, in the brains of Drosophila larvae it is the old centrosome that is instead maintained by the differentiating ganglion cell, which in this particular case shows a longer lifespan than the self-renewing neuroblast [Citation136]. Therefore, based on the widespread conservation of preestablished MTOC distribution patterns during the asymmetric division of cells from many organisms, and although it still remains as a controversial issue whether stem cells transmit aging factors to their daughters, the abovementioned results open a new and exciting research field to explore with regards to stem cell aging.
Final considerations
The spindle MTOCs orchestrate the formation of the bipolar array of microtubules that serves as the main platform for the astonishing and complex machinery that facilitates the segregation of the chromosomes during cell division. However, beyond this already remarkable task, these structures play additional fundamental roles in the regulation of cell cycle progression, checkpoint signaling, nutrient sensing, cellular transport and replicative aging. All these critical functions turn SPBs and centrosomes into core signaling hubs where multiple pathways converge and are coordinated to regulate a plethora of cellular processes, as well as into “guiding torches” that provide spatial cues and facilitate the generation of asymmetry during cell division. The paramount contribution of the spindle MTOCs to the regulation of such a variety of cellular processes makes these fascinating structures an especially attractive focus where to center our future research.
Author Contributions
A.M.R. and F.M.-C. wrote the manuscript.
Acknowledgments
We thank members of the Monje-Casas’ group for critical reading of the manuscript. Research in the Monje-Casas’ laboratory was supported by the European Union (FEDER) and the Spanish Ministry of Science, Innovation and Universities (Grants BFU2016-76642-P and BFU2017-92284-EXP).
Disclosure statement
The authors declare no competing interests.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Prosser SL, Pelletier L. Mitotic spindle assembly in animal cells: a fine balancing act. Nat Rev Mol Cell Biol. 2017;18(3):187–201.
- Winey M, Bloom K. Mitotic spindle form and function. Genetics. 2012;190:1197–1224.
- Meraldi P. Centrosomes in spindle organization and chromosome segregation: a mechanistic view. Chromosome Res. 2016;24:19–34.
- Wu J, Akhmanova A. Microtubule-organizing centers. Annu Rev Cell Dev Biol. 2017;33:51–75.
- Valerio-Santiago M, Monje-Casas F. Tem1 localization to the spindle pole bodies is essential for mitotic exit and impairs spindle checkpoint function. J Cell Biol. 2011;192:599–614.
- Baro B, Queralt E, Monje-Casas F. Regulation of mitotic exit in saccharomyces cerevisiae. Methods Mol Biol. 2017;1505:3–17.
- Visintin R, Hwang ES, Amon A. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature. 1999;398:818–823.
- Shou W, Seol JH, Shevchenko A, et al. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell. 1999;97:233–244.
- Visintin R, Amon A. Regulation of the mitotic exit protein kinases Cdc15 and Dbf2. Mol Biol Cell. 2001;12:2961–2974.
- Magidson V, Chang F, Khodjakov A. Regulation of cytokinesis by spindle-pole bodies. Nat Cell Biol. 2006;8(8):891–893.
- Elserafy M, Saric M, Neuner A, et al. Molecular mechanisms that restrict yeast centrosome duplication to one event per cell cycle. Curr Biol. 2014;24:1456–1466.
- Lengefeld J, Hotz M, Rollins M, et al. Budding yeast wee1 distinguishes spindle pole bodies to guide their pattern of age-dependent segregation. Nat Cell Biol. 2017;19:941–951.
- Tamborrini D, Juanes MA, Ibanes S, et al. Recruitment of the mitotic exit network to yeast centrosomes couples septin displacement to actomyosin constriction. Nat Commun. 2018;9:4308.
- Powers BL, Hall MC. Re-examining the role of Cdc14 phosphatase in reversal of Cdk phosphorylation during mitotic exit. J Cell Sci. 2017;130:2673–2681.
- Shirk K, Jin H, Giddings TH Jr., et al. The Aurora kinase Ipl1 is necessary for spindle pole body cohesion during budding yeast meiosis. J Cell Sci. 2011;124:2891–2896.
- Kim S, Meyer R, Chuong H, et al. Dual mechanisms prevent premature chromosome segregation during meiosis. Genes Dev. 2013;27:2139–2146.
- Nakamura TS, Numajiri Y, Okumura Y, et al. Dynamic localization of a yeast development-specific PP1 complex during prospore membrane formation is dependent on multiple localization signals and complex formation. Mol Biol Cell. 2017;28:3881–3895.
- Hinchcliffe EH, Miller FJ, Cham M, et al. Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science. 2001;291:1547–1550.
- Khodjakov A, Rieder CL. Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J Cell Biol. 2001;153:237–242.
- Matsumoto Y, Maller JL. A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry. Science. 2004;306:885–888.
- Pascreau G, Eckerdt F, Churchill ME, et al. Discovery of a distinct domain in cyclin A sufficient for centrosomal localization independently of Cdk binding. Proc Natl Acad Sci U S A. 2010;107:2932–2937.
- Krämer A, Mailand N, Lukas C, et al. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat Cell Biol. 2004;6:884–891.
- Hirota T, Kunitoku N, Sasayama T, et al. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114:585–598.
- Golsteyn RM, Mundt KE, Fry AM, et al. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J Cell Biol. 1995;129:1617–1628.
- Holland AJ, Fachinetti D, Da Cruz S, et al. Polo-like kinase 4 controls centriole duplication but does not directly regulate cytokinesis. Mol Biol Cell. 2012;23:1838–1845.
- Bruinsma W, Raaijmakers JA, Medema RH. Switching polo-like kinase-1 on and off in time and space. Trends Biochem Sci. 2012;37:534–542.
- Schmucker S, Sumara I. Molecular dynamics of PLK1 during mitosis. Mol Cell Oncol. 2014;1:e954507.
- Macurek L, Lindqvist A, Lim D, et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455:119–123.
- Seki A, Coppinger JA, Jang CY, et al. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–1658.
- Bruinsma W, Aprelia M, Kool J, et al. Spatial separation of Plk1 phosphorylation and activity. Front Oncol. 2015;5:132.
- Rapley J, Baxter JE, Blot J, et al. Coordinate regulation of the mother centriole component nlp by nek2 and plk1 protein kinases. Mol Cell Biol. 2005;25:1309–1324.
- Mardin BR, Agircan FG, Lange C, et al. Plk1 controls the Nek2A-PP1gamma antagonism in centrosome disjunction. Curr Biol. 2011;21:1145–1151.
- Kiyomitsu T, Cheeseman IM. Chromosome- and spindle-pole-derived signals generate an intrinsic code for spindle position and orientation. Nat Cell Biol. 2012;14:311–317.
- Kratz AS, Barenz F, Richter KT, et al. Plk4-dependent phosphorylation of STIL is required for centriole duplication. Biol Open. 2015;4:370–377.
- Ohta M, Ashikawa T, Nozaki Y, et al. Direct interaction of Plk4 with STIL ensures formation of a single procentriole per parental centriole. Nat Commun. 2014;5:5267.
- Bury L, Coelho PA, Simeone A, et al. Plk4 and aurora A cooperate in the initiation of acentriolar spindle assembly in mammalian oocytes. J Cell Biol. 2017;216:3571–3590.
- Coelho PA, Bury L, Sharif B, et al. Spindle formation in the mouse embryo requires Plk4 in the absence of centrioles. Dev Cell. 2013;27:586–597.
- Ohta M, Watanabe K, Ashikawa T, et al. Bimodal binding of STIL to Plk4 controls proper centriole copy number. Cell Rep. 2018;23:3160–9 e4.
- Cunha-Ferreira I, Rodrigues-Martins A, Bento I, et al. The SCF/Slimb ubiquitin ligase limits centrosome amplification through degradation of SAK/PLK4. Curr Biol. 2009;19:43–49.
- Holland AJ, Lan W, Niessen S, et al. Polo-like kinase 4 kinase activity limits centrosome overduplication by autoregulating its own stability. J Cell Biol. 2010;188:191–198.
- Montenegro Gouveia S, Zitouni S, Kong D, et al. PLK4 is a microtubule-associated protein that self-assembles promoting de novo MTOC formation. J Cell Sci. 2018;132(4):jcs219501.
- Song MH, Liu Y, Anderson DE, et al. Protein phosphatase 2A-SUR-6/B55 regulates centriole duplication in C. elegans by controlling the levels of centriole assembly factors. Dev Cell. 2011;20:563–571.
- Kitagawa D, Fluckiger I, Polanowska J, et al. PP2A phosphatase acts upon SAS-5 to ensure centriole formation in C. elegans embryos. Dev Cell. 2011;20:550–562.
- Enos SJ, Dressler M, Gomes BF, et al. Phosphatase PP2A and microtubule-mediated pulling forces disassemble centrosomes during mitotic exit. Biol Open. 2018;7(1): bio029777.
- Boudreau V, Chen R, Edwards A, et al. PP2A-B55/SUR-6 collaborates with the nuclear lamina for centrosome separation during mitotic entry. Mol Biol Cell. 2019;30:876–886.
- Wlodarchak N, Xing Y. PP2A as a master regulator of the cell cycle. Crit Rev Biochem Mol Biol. 2016;51:162–184.
- Peel N, Iyer J, Naik A, et al. Protein phosphatase 1 down regulates ZYG-1 levels to limit centriole duplication. PLoS Genet. 2017;13:e1006543.
- Joukov V, Walter JC, De Nicolo A. The Cep192-organized aurora A-Plk1 cascade is essential for centrosome cycle and bipolar spindle assembly. Mol Cell. 2014;55:578–591.
- Nasa I, Trinkle-Mulcahy L, Douglas P, et al. Recruitment of PP1 to the centrosomal scaffold protein CEP192. Biochem Biophys Res Commun. 2017;484:864–870.
- Sankaran S, Crone DE, Palazzo RE, et al. Aurora-A kinase regulates breast cancer associated gene 1 inhibition of centrosome-dependent microtubule nucleation. Cancer Res. 2007;67:11186–11194.
- Meraldi P, Nigg EA. Centrosome cohesion is regulated by a balance of kinase and phosphatase activities. J Cell Sci. 2001;114:3749–3757.
- Saito RM, Perreault A, Peach B, van den Heuvel S, et al. The CDC-14 phosphatase controls developmental cell-cycle arrest in C. elegans. Nat Cell Biol. 2004;6:777–783.
- Kaiser BK, Zimmerman ZA, Charbonneau H, et al. Disruption of centrosome structure, chromosome segregation, and cytokinesis by misexpression of human Cdc14A phosphatase. Mol Biol Cell. 2002;13:2289–2300.
- Mocciaro A, Berdougo E, Zeng K, et al. Vertebrate cells genetically deficient for Cdc14A or Cdc14B retain DNA damage checkpoint proficiency but are impaired in DNA repair. J Cell Biol. 2010;189:631–639.
- Mailand N, Lukas C, Kaiser BK, et al. Deregulated human Cdc14A phosphatase disrupts centrosome separation and chromosome segregation. Nat Cell Biol. 2002;4:317–322.
- Clément A, Solnica-Krezel L, Gould KL. Functional redundancy between Cdc14 phosphatases in zebrafish ciliogenesis. Dev Dyn. 2012;241:1911–1921.
- Schindler K, Schultz RM. The CDC14A phosphatase regulates oocyte maturation in mouse. Cell Cycle. 2009;8:1090–1098.
- Kaiser BK, Nachury MV, Gardner BE, et al. Xenopus Cdc14 alpha/beta are localized to the nucleolus and centrosome and are required for embryonic cell division. BMC Cell Biol. 2004;5:27.
- Krasinska L, de Bettignies G, Fisher D, et al. Regulation of multiple cell cycle events by Cdc14 homologues in vertebrates. Exp Cell Res. 2007;313:1225–1239.
- Gromley A, Jurczyk A, Sillibourne J, et al. A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J Cell Biol. 2003;161:535–545.
- Lanz MC, Dibitetto D, Smolka MB. DNA damage kinase signaling: checkpoint and repair at 30 years. Embo J. 2019;38:e101801.
- Su TT. Cellular responses to DNA damage: one signal, multiple choices. Annu Rev Genet. 2006;40:187–208.
- Villoria MT, Ramos F, Duenas E, et al. Stabilization of the metaphase spindle by Cdc14 is required for recombinational DNA repair. Embo J. 2017;36:79–101.
- Pereira G, Hofken T, Grindlay J, et al. The Bub2p spindle checkpoint links nuclear migration with mitotic exit. Mol Cell. 2000;6:1–10.
- Geymonat M, Spanos A, Smith SJ, et al. Control of mitotic exit in budding yeast. in vitro regulation of Tem1 GTPase by Bub2 and Bfa1. J Biol Chem. 2002;277:28439–28445.
- Hu F, Wang Y, Liu D, et al. Regulation of the Bub2/Bfa1 GAP complex by Cdc5 and cell cycle checkpoints. Cell. 2001;107:655–665.
- Valerio-Santiago M, de Los Santos-velazquez AI, Monje-Casas F. Inhibition of the mitotic exit network in response to damaged telomeres. PLoS Genet. 2013;9:e1003859.
- Pereira G, Schiebel E. Kin4 kinase delays mitotic exit in response to spindle alignment defects. Mol Cell. 2005;19:209–221.
- D’Aquino KE, Monje-Casas F, Paulson J, et al. The protein kinase Kin4 inhibits exit from mitosis in response to spindle position defects. Mol Cell. 2005;19:223–234.
- Bardin AJ, Visintin R, Amon A. A mechanism for coupling exit from mitosis to partitioning of the nucleus. Cell. 2000;102:21–31.
- Monje-Casas F, Amon A. Cell polarity determinants establish asymmetry in MEN signaling. Dev Cell. 2009;16:132–145.
- Caydasi AK, Pereira G. Spindle alignment regulates the dynamic association of checkpoint proteins with yeast spindle pole bodies. Dev Cell. 2009;16:146–156.
- Bertazzi DT, Kurtulmus B, Pereira G. The cortical protein Lte1 promotes mitotic exit by inhibiting the spindle position checkpoint kinase Kin4. J Cell Biol. 2011;193:1033–1048.
- Falk JE, Chan LY, Amon A. Lte1 promotes mitotic exit by controlling the localization of the spindle position checkpoint kinase Kin4. Proc Natl Acad Sci U S A. 2011;108:12584–12590.
- Chan LY, Amon A. Spindle position is coordinated with cell-cycle progression through establishment of mitotic exit-activating and -inhibitory zones. Mol Cell. 2010;39:444–454.
- Serrano D, D’Amours D. When genome integrity and cell cycle decisions collide: roles of polo kinases in cellular adaptation to DNA damage. Syst Synth Biol. 2014;8:195–203.
- Toczyski DP, Galgoczy DJ, Hartwell LH. CDC5 and CKII control adaptation to the yeast DNA damage checkpoint. Cell. 1997;90:1097–1106.
- Rawal CC, Riccardo S, Pesenti C, et al. Reduced kinase activity of polo kinase Cdc5 affects chromosome stability and DNA damage response in S. cerevisiae. Cell Cycle. 2016;15:2906–2919.
- Ratsima H, Serrano D, Pascariu M, et al. Centrosome-dependent bypass of the dna damage checkpoint by the polo kinase Cdc5. Cell Rep. 2016;14:1422–1434.
- Löffler H, Fechter A, Liu FY, et al. DNA damage-induced centrosome amplification occurs via excessive formation of centriolar satellites. Oncogene. 2013;32:2963–2972.
- Roninson IB, Broude EV, Chang BD. If not apoptosis, then what? treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist Updat. 2001;4:303–313.
- Takada S, Kelkar A, Theurkauf WE. Drosophila checkpoint kinase 2 couples centrosome function and spindle assembly to genomic integrity. Cell. 2003;113:87–99.
- Hut HM, Lemstra W, Blaauw EH, et al. Centrosomes split in the presence of impaired DNA integrity during mitosis. Mol Biol Cell. 2003;14:1993–2004.
- Smits VA, Klompmaker R, Arnaud L, et al. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat Cell Biol. 2000;2:672–676.
- Bruinsma W, Aprelia M, Garcia-Santisteban I, et al. Inhibition of Polo-like kinase 1 during the DNA damage response is mediated through loss of aurora A recruitment by Bora. Oncogene. 2017;36:1840–1848.
- Krystyniak A, Garcia-Echeverria C, Prigent C, et al. Inhibition of Aurora A in response to DNA damage. Oncogene. 2006;25:338–348.
- Qin B, Gao B, Yu J, et al. Ataxia telangiectasia-mutated- and Rad3-related protein regulates the DNA damage-induced G2/M checkpoint through the Aurora A cofactor Bora protein. J Biol Chem. 2013;288:16139–16144.
- Chan EH, Santamaria A, Sillje HH, et al. Plk1 regulates mitotic Aurora A function through betaTrCP-dependent degradation of hBora. Chromosoma. 2008;117:457–469.
- Tavernier N, Noatynska A, Panbianco C, et al. Cdk1 phosphorylates SPAT-1/Bora to trigger PLK-1 activation and drive mitotic entry in C. elegans embryo. J Cell Biol. 2015;208:661–669.
- Yata K, Lloyd J, Maslen S, et al. Plk1 and CK2 act in concert to regulate Rad51 during DNA double strand break repair. Mol Cell. 2012;45:371–383.
- Mi J, Guo C, Brautigan DL, et al. Protein phosphatase-1alpha regulates centrosome splitting through Nek2. Cancer Res. 2007;67:1082–1089.
- Zhang W, Fletcher L, Muschel RJ. The role of Polo-like kinase 1 in the inhibition of centrosome separation after ionizing radiation. J Biol Chem. 2005;280:42994–42999.
- Petersen J, Nurse P. TOR signalling regulates mitotic commitment through the stress MAP kinase pathway and the Polo and Cdc2 kinases. Nat Cell Biol. 2007;9:1263–1272.
- Messier V, Zenklusen D, Michnick SW. A nutrient-responsive pathway that determines M phase timing through control of B-cyclin mRNA stability. Cell. 2013;153:1080–1093.
- Nakashima A, Maruki Y, Imamura Y, et al. The yeast Tor signaling pathway is involved in G2/M transition via polo-kinase. PLoS One. 2008;3:e2223.
- Searle JS, Schollaert KL, Wilkins BJ, et al. The DNA damage checkpoint and PKA pathways converge on APC substrates and Cdc20 to regulate mitotic progression. Nat Cell Biol. 2004;6:138–145.
- Chauhan N, Visram M, Cristobal-Sarramian A, et al. Morphogenesis checkpoint kinase swe1 is the executor of lipolysis-dependent cell-cycle progression. Proc Natl Acad Sci U S A. 2015;112:E1077–85.
- Cuyas E, Corominas-Faja B, Joven J, et al. Cell cycle regulation by the nutrient-sensing mammalian target of rapamycin (mTOR) pathway. Methods Mol Biol. 2014;1170:113–144.
- Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The active form of the metabolic sensor: AMP-activated protein kinase (AMPK) directly binds the mitotic apparatus and travels from centrosomes to the spindle midzone during mitosis and cytokinesis. Cell Cycle. 2009;8:2385–2398.
- Vazquez-Martin A, Oliveras-Ferraros C, Cufi S, et al. Polo-like kinase 1 regulates activation of AMP-activated protein kinase (AMPK) at the mitotic apparatus. Cell Cycle. 2011;10(8):1295–1302.
- Li Q-R, Yan X-M, Guo L, et al. AMPK regulates anaphase central spindle length by phosphorylation of KIF4A. J Mol Cell Biol. 2018;10(1):2–17.
- Platani M, Trinkle-Mulcahy L, Porter M, et al. Mio depletion links mTOR regulation to Aurora A and Plk1 activation at mitotic centrosomes. J Cell Biol. 2015;210(1):45–62.
- Vandame P, Spriet C, Trinel D, et al. The spatio-temporal dynamics of PKA activity profile during mitosis and its correlation to chromosome segregation. Cell Cycle. 2014;13:3232–3240.
- Noree C, Monfort E, Shotelersuk V. Human asparagine synthetase associates with the mitotic spindle. Biol Open. 2018;7.
- Culver C, Melvin A, Mudie S, et al. HIF-1alpha depletion results in SP1-mediated cell cycle disruption and alters the cellular response to chemotherapeutic drugs. Cell Cycle. 2011;10:1249–1260.
- Hubbi ME, Kshitiz, Gilkes DM, et al. A nontranscriptional role for HIF-1alpha as a direct inhibitor of DNA replication. Sci Signal. 2013;6:ra10.
- Strowitzki MJ, Cummins EP, Taylor CT. Protein hydroxylation by hypoxia-inducible factor (HIF) hydroxylases: unique or ubiquitous? Cells. 2019;8.
- Moser SC, Bensaddek D, Ortmann B, et al. PHD1 links cell-cycle progression to oxygen sensing through hydroxylation of the centrosomal protein Cep192. Dev Cell. 2013;26:381–392.
- Kim S, Dynlacht BD. Centrosomes tune in to metabolic state and turn on to oxygen. Dev Cell. 2013;26:325–326.
- Mihajlovic AI, FitzHarris G. Segregating chromosomes in the mammalian oocyte. Curr Biol. 2018;28:R895–R907.
- Jansen RP. RNA-cytoskeletal associations. Faseb J. 1999;13:455–466.
- Ruzanov PV, Evdokimova VM, Korneeva NL, et al. Interaction of the universal mRNA-binding protein, p50, with actin: a possible link between mRNA and microfilaments. J Cell Sci. 1999;112(Pt 20):3487–3496.
- Groisman I, Huang YS, Mendez R, et al. CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division. Cell. 2000;103:435–447.
- Lambert JD, Nagy LM. Asymmetric inheritance of centrosomally localized mRNAs during embryonic cleavages. Nature. 2002;420:682–686.
- Kingsley EP, Chan XY, Duan Y, et al. Widespread RNA segregation in a spiralian embryo. Evol Dev. 2007;9:527–539.
- Lecuyer E, Yoshida H, Parthasarathy N, et al. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell. 2007;131:174–187.
- Yaffe MP, Stuurman N, Vale RD. Mitochondrial positioning in fission yeast is driven by association with dynamic microtubules and mitotic spindle poles. Proc Natl Acad Sci U S A. 2003;100:11424–11428.
- Fisk HA, Yaffe MP. Mutational analysis of Mdm1p function in nuclear and mitochondrial inheritance. J Cell Biol. 1997;138:485–494.
- Dalton CM, Carroll J. Biased inheritance of mitochondria during asymmetric cell division in the mouse oocyte. J Cell Sci. 2013;126:2955–2964.
- Katayama M, Zhong Z, Lai L, et al. Mitochondrial distribution and microtubule organization in fertilized and cloned porcine embryos: implications for developmental potential. Dev Biol. 2006;299:206–220.
- Knabe W, Kuhn HJ. The role of microtubules and microtubule-organising centres during the migration of mitochondria. J Anat. 1996;189(Pt 2):383–391.
- Maccari I, Zhao R, Peglow M, et al. Cytoskeleton rotation relocates mitochondria to the immunological synapse and increases calcium signals. Cell Calcium. 2016;60:309–321.
- Morlino G, Barreiro O, Baixauli F, et al. Miro-1 links mitochondria and microtubule Dynein motors to control lymphocyte migration and polarity. Mol Cell Biol. 2014;34:1412–1426.
- Ogbadoyi EO, Robinson DR, Gull K. A high-order trans-membrane structural linkage is responsible for mitochondrial genome positioning and segregation by flagellar basal bodies in trypanosomes. Mol Biol Cell. 2003;14:1769–1779.
- Van Blerkom J. Microtubule mediation of cytoplasmic and nuclear maturation during the early stages of resumed meiosis in cultured mouse oocytes. Proc Natl Acad Sci U S A. 1991;88:5031–5035.
- Sutterlin C, Colanzi A. The Golgi and the centrosome: building a functional partnership. J Cell Biol 2010; 188:621–8
- Tormanen K, Ton C, Waring BM, et al. Function of golgi-centrosome proximity in RPE-1 cells. PLoS One. 2019;14:e0215215.
- Orlofsky A. Positioning of the centrosome and golgi complex. Results Probl Cell Differ. 2019;67:127–200.
- Saraste J, Prydz K. A new look at the functional organization of the golgi ribbon. Front Cell Dev Biol. 2019;7:171.
- Hehnly H, Chen CT, Powers CM, et al. The centrosome regulates the Rab11- dependent recycling endosome pathway at appendages of the mother centriole. Curr Biol. 2012;22:1944–1950.
- Brose L, Crest J, Tao L, et al. Polo kinase mediates the phosphorylation and cellular localization of Nuf/FIP3, a Rab11 effector. Mol Biol Cell. 2017;28:1435–1443.
- Pelletier L, Yamashita YM. Centrosome asymmetry and inheritance during animal development. Curr Opin Cell Biol. 2012;24:541–546.
- Pereira G, Tanaka TU, Nasmyth K, et al. Modes of spindle pole body inheritance and segregation of the Bfa1p-Bub2p checkpoint protein complex. Embo J. 2001;20:6359–6370.
- Yamashita YM, Mahowald AP, Perlin JR, et al. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–521.
- Salzmann V, Chen C, Chiang CY, et al. Centrosome-dependent asymmetric inheritance of the midbody ring in Drosophila germline stem cell division. Mol Biol Cell. 2014;25:267–275.
- Januschke J, Llamazares S, Reina J, et al. Drosophila neuroblasts retain the daughter centrosome. Nat Commun. 2011;2:243.
- Izumi H, Kaneko Y. Evidence of asymmetric cell division and centrosome inheritance in human neuroblastoma cells. Proc Natl Acad Sci U S A. 2012;109:18048–18053.
- Wang X, Tsai JW, Imai JH, et al. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature. 2009;461:947–955.
- Manzano-Lopez J, Matellan L, Alvarez-Llamas A, et al. Asymmetric inheritance of spindle microtubule-organizing centres preserves replicative lifespan. Nat Cell Biol. 2019;21:952–965.
- Denoth Lippuner A, Julou T, Barral Y. Budding yeast as a model organism to study the effects of age. FEMS Microbiol Rev. 2014;38:300–325.
- Cheng J, Turkel N, Hemati N, et al. Centrosome misorientation reduces stem cell division during ageing. Nature. 2008;456:599–604.
