ABSTRACT
Budding yeast, Saccharomyces cerevisiae, has been widely used as a model system to study cellular signaling in response to internal and external cues. Yeast was among the first organisms in which the architecture, feedback mechanisms and physiological responses of various MAP kinase signaling cascades were studied in detail. Although these MAP kinase pathways are activated by different signals and elicit diverse cellular responses, such as adaptation to stress and mating, they function as an interconnected signaling network, as they influence each other and, in some cases, even share components. Indeed, various stress signaling pathways interfere with pheromone signaling that triggers a distinct cellular differentiation program. However, the molecular mechanisms responsible for this crosstalk are still poorly understood. Here, we review the general topology of the yeast MAP kinase signaling network and highlight recent and new data revealing how conflicting intrinsic and extrinsic signals are interpreted to orchestrate appropriate cellular responses.
KEYWORDS:
The yeast MAP kinase signaling network
Budding yeast comprises five MAP kinase pathways that respond to diverse external and internal conditions to adapt cellular behavior ([Citation1,Citation2], ). For example, MAP kinase pathways coordinate cellular protective mechanisms during various stress conditions, including osmolar-, oxidative-, and physical stress [Citation3–Citation5]. Moreover, MAP-kinase pathways also orchestrate cell differentiation such as mating of haploid cells to opposite mating type (Mat a and Mat α) [Citation6] or induction of meiosis in diploid cells starved of nutrients [Citation7]. Even with shared common components between some MAP kinase pathways, cells faithfully respond to specific triggers and orchestrate unique downstream responses. Moreover, since cells are more vulnerable to lysis at several stages of their life cycle, stress signaling pathways interfere with cell division and differentiation processes. For example, yeast mating is prevented when cells are exposed to high osmolarity or mechanical stress conditions. However, the molecular mechanisms underlying insulation and crosstalk of MAP kinase pathways remain poorly understood. Importantly, recent work identified the sensors and intracellular signaling pathways activated by mechanical stress [Citation8,Citation9] and also revealed how these pathways interfere with yeast mating [Citation10]. Below, we will first introduce different yeast MAP kinase pathways and then describe how they functionally interact with each other.
Figure 1. Overview of the CWI, pheromone response and HOG MAP kinase cascades in yeast.
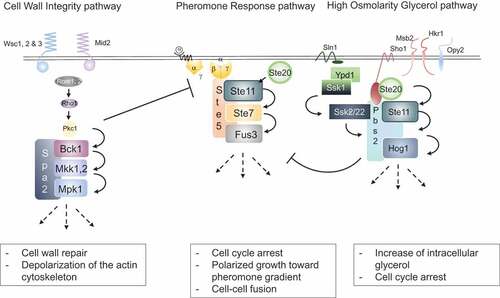
The mating pheromone pathway is initiated when haploid yeast cells from the opposite mating type (Mat a and Mat α) encounter each other. Cells secrete either a- or α-pheromones that bind to G-protein coupled receptors on cells of the opposite mating type (, center) [Citation11]. This in turn leads to dissociation of the α-subunit of the trimeric G-protein, and βγ heterodimers recruit the scaffolds Ste5 and Far1 to the plasma membrane by binding to their RING-H2 domains. Far1 then binds various effector proteins including Bem1 and Cdc24, a GEF for the Rho-type GTPase Cdc42, which collectively mediate polarized growth toward the mating partner [Citation12–Citation14]. Likewise, activated Cdc42 and Ste5 recruit the PAK-like kinase Ste20 and the MAP kinase module consisting of the MEKK Ste11, the MEK Ste7 and the MAPK Fus3 [Citation15]. Activated Fus3 phosphorylates multiple substrates orchestrating the different cellular activities required for the complex mating process, including induction of a transcriptional program, cell cycle arrest in G1, oriented polarized growth and cell-cell and nuclear fusion.
The high osmolarity glycerol (HOG) pathway protects cells from lysis during salt stress and other conditions with a high external osmolarity. Two osmo-sensors, Sho1 and Sln1, located at the cell membrane are able to activate separate branches of the HOG pathway (, right). Activated Sho1 recruits Pbs2, which acts both as a MEK to phosphorylate the MAP kinase Hog1 and as a scaffold recruiting other upstream kinases including its own activator Ste11. Additionally, the three transmembrane proteins Msb2, Hrk1 and Opy2 are needed for full Ste11 activation by recruiting various co-stimulators to the cell membrane [Citation16,Citation17]. The partially redundant Sln1 branch uses a histidine phospho-relay system, which inhibits the kinase Ssk1in absence of osmotic stress through the intermediate histidine phosphate transfer protein Ypd1 [Citation18]. Upon Sln1 activation in response to osmotic stress, Ssk1 is dephosphorylated and activated, in turn activating its downstream MEKKs Ssk2 and Ssk22. Like Ste11, Ssk2 and Ssk22 interact and phosphorylate Pbs2 leading to Hog1 activation resulting in an increase of intracellular glycerol concentrations, cell cycle arrest and cellular adaptation.
Finally, multiple forms of cell wall stress, including hypo-osmolarity and several forms of chemical and physical insult result in activation of the cell wall integrity (CWI) pathway. Different membrane-anchored sensors activate the GEFs Rom1 and Rom2, which in turn stimulate the GTPase Rho1 (, left). GTP-bound Rho1 recruits Pkc1, which in turn activates a MAP kinase module composed of the MEKK Bck1, the two closely-related MEKs, Mkk1 and Mkk2 and the MAP kinase Mpk1 (Slt2) [Citation19]. The scaffold protein Spa2 recruits these components to sites of polarized growth to facilitate cell wall remodeling [Citation20].
Mechanisms that ensure pathway specificity of MAP kinase pathways
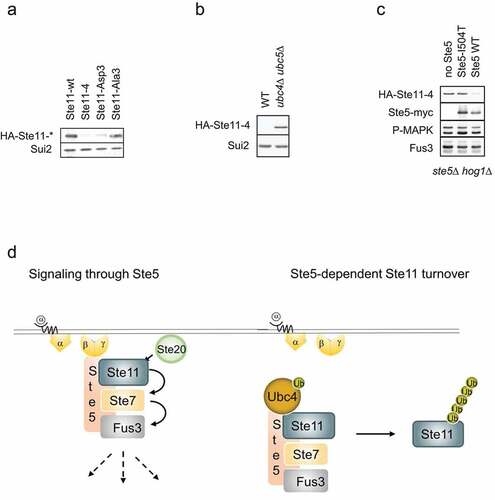
The revelation that Ste11 functions in both the HOG- and the pheromone response pathways with shared upstream activators but different downstream targets and distinct physiological consequences raised the question of how pathway specificity is ensured. The molecular mechanism underlying Ste11 activation is identical regardless if the upstream signal is pheromone or high osmolarity. In both cases, the PAK-like kinase Ste20 phosphorylates several residues within a Ste11 N-terminal motif, thereby relieving autoinhibition by the C-terminal catalytic domain. Importantly, in contrast to Ste11 activation by their cognate signals, cells expressing constitutively-active Ste11 mutants, either mimicking phosphorylation of the activating residues (Ste11-Asp3) or preventing autoinhibition by other means (Ste11-4) [Citation21], activate both the Hog1 and Fus3 MAP kinases, and as a result induce mating- and high-osmolarity specific responses [Citation22]. Thus, active Ste11 is able in principle to simultaneously activate the different kinases and downstream responses, but this expansion is prevented with a physiological activation of the pathways.
Interestingly, Ste11 is degraded by proteasome-dependent mechanisms upon prolonged pheromone induction [Citation23]. Indeed, when comparing cells expressing wild type, non-activatable and constitutively-active Ste11 mutants, we realized that the steady-state protein levels of the mutants that activate pheromone signaling are strongly reduced compared to wild type Ste11 or a non-activatable mutant (). In contrast, the levels of non-phosphorylatable Ste11 were increased compared to wild type controls [Citation22]. Scaffold proteins spatially and temporally sequester components of MAP kinase modules and thereby contribute to pathway specificity and insulation. The scaffold Ste5 possesses a folded RING-H2 domain [Citation24], which in E3 ubiquitin-ligases binds E2 enzymes and catalyzes the transfer of activated ubiquitin to lysine residues of bound substrates. Indeed, Ste5 was previously shown to bind the E2 ubiquitin-conjugating enzyme Ubc4 [Citation25], and the turnover of active Ste11 is dependent on the presence of the two functionally redundant E2s Ubc4 and Ubc5 (). Together, these results indicate that Ste5 may function as a ubiquitin-ligase to trigger ubiquitin-dependent degradation of active Ste11. To test this hypothesis, we compared HA-Ste11-4 [Citation21] levels in ste5Δ cells harboring either an empty control plasmid or plasmids expressing from the endogenous promoter either wild type Ste5 or the Ste5-I504T mutant [Citation26] unable to interact with Ste11. Strikingly, although Fus3 was activated in all cases, Ste11 degradation was dependent on Ste5 (), implying that Ste5 functions as a E3 ligase to target bound Ste11 for ubiquitin-dependent turnover. This result raises the possibility that pathway insulation in response to pheromones is achieved by Ste5-dependent degradation of bound Ste11, thereby preventing that activated Ste11 dissociates and inappropriately activates other MAP kinase modules (). Further work is required to test this exciting possibility. If correct, it will also be interesting to test whether analogous mechanisms prevent that Ste11 activates Fus3 in cells exposed to high osmolarity. Indeed, in contrast to wild type, the pheromone response pathway is activated in hog1Δ cells exposed to high osmolarity conditions, implying that Hog1 activity prevents spurious activation of the pheromone pathway [Citation27]. This cross-activation depends on the presence of the osmosensor Sho1 and the pheromone pathway components Ste20, Ste11 and Ste7, but does not require the scaffold Ste5. Thus, if Ste11 degradation indeed contributes to pathway insulation, an unknown E3-ligase is likely to function during HOG signaling. Additionally, the polarity scaffold Far1, also recruited to free Gβγ during pheromone signaling, contains a RING-H2 domain homologous to the RING-H2 domain in Ste5, and might in an analogous fashion be involved in the turnover of the polarity protein Cdc24.
Figure 2. Steady-state levels of active Ste11 is dependent on Ste5 and Ubc4/5.
Some crosstalk mechanisms interfere with membrane-recruitment of Ste5 to prevent untimely pheromone signaling
While individual MAP kinase pathways are activated by specific stimuli or stress conditions simultaneously, the overall behavior needs to be carefully modulated to avoid a competing and potentially self-destructive response. For example, cells are highly vulnerable during distinct cell cycle stages, extended periods of polarized growth, or cell-cell fusion during mating, and thus bud emergence and mating should not be executed during intrinsic and extrinsic stress conditions such as nutrient limitation, osmotic or physical stress. Pioneering work by the Pryciak laboratory demonstrated how pheromone signaling is prevented in cells that are committed to divide with activated G1 cyclin-dependent kinase complex (G1 Cdk) to initiate polarized growth and bud emergence [Citation28]. Interestingly, the G1 Cdk phosphorylates several sites within the amino terminal domain of the Ste5 scaffold, which in turn prevents its recruitment to the plasma membrane and thereby blocks Fus3 activation even in the presence of pheromones. Thus, cross talk between the cell cycle machinery and pheromone signaling is controlled by phosphorylation-dependent regulation of Ste5 membrane recruitment. The same Ste5 sites are also phosphorylated by Fus3, constituting a negative feedback mechanism that limits prolonged pheromone signaling [Citation29].
While the mechanism of this intrinsic crosstalk is well understood, how external stress conditions prevent activation of the pheromone pathway was only recently described in the context of mechanical stress. Increasing pressure by overgrowth of yeast cells in space-restricted chambers demonstrated activation of the MEKK Ste11 through the osmo-receptors Sho1 and Msb2 [Citation30,Citation31]. It remains unclear which particular MAP kinase acts downstream of Ste11 to protect cells from lysis, but interesting double mutants show increased cell death under these conditions [Citation8].
In addition, a recent study unraveled that Ca2+ influx and activation of the CWI signaling pathway ensure survival during compressive mechanical stress [Citation9]. The device developed for this study relies on the deformation of PDMS upon an increase in air pressure causing micro-pillars to physically trap and apply compressive mechanical force to cells. Physical stress opens the calcium-channel composed of Mid1 and Cch1, and thus activates the Ca2+/calcineurin pathway to regulate cytoskeletal dynamics and Pkc1 activity. Moreover, the transmembrane protein Mid2 was shown to function as a cellular sensor for compressive stress, which similar to Wsc1, activates the GEFs Rom1 and Rom2, leading to the formation of Rho1-GTP and activation of Pkc1 (). Indeed, upon mechanical stress, Pkc1 is recruited to the cell membrane in a Mid2 dependent manner. Pkc1 acts upstream of the Mpk1 MAP kinase module, and activated Mpk1 depolarizes the actin cytoskeleton to prevent polarized growth. Consistent with this notion, cell lysis of mid2Δ cells exposed to compressive stress could be overcome by chemical inhibition of actin polymerization [Citation9]. Similar results were also reported in S. pombe, where Wsc1 functions as a mechanical sensor monitoring cell wall homeostasis and probing physical properties by activating a MAP kinase module [Citation32].
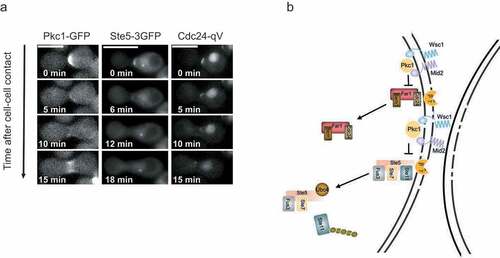
Interestingly, budding yeast cells exposed to mechanical stress block activation of the mating pathway, thereby preventing lysis during shmoo formation. This crosstalk is mediated by Pkc1, which phosphorylates the scaffolds Ste5 and Far1 in their RING-H2 domains, thereby blocking the interaction with Gβγ (). Thus, analogous to the cell cycle regulation described above, mechanical stress prevents membrane recruitment of Ste5, but the underlying mechanisms are distinct. While it remains unclear how phosphorylation of a single site in the RING-H2 domain of Ste5 blocks it’s signaling function by interfering with Gβγ binding, this regulatory mechanism is expected to function as an on-off switch characteristic for rapid stress responses rather than a tunable-rheostat typically observed for multisite phosphorylation.
In addition to extrinsic mechanical stress, Pkc1-dependent crosstalk also operates during zygote formation, in which mechanical pressure seems to occur as a result of cell-cell fusion. Consistent with this notion, both Ste5 and Pkc1 initially accumulate at cell-cell contact sites, where Pkc1 triggers rapid removal of Ste5 to down-tune pheromone signaling (, Citation10). Interestingly, mid2Δ cells show increased cell death during mating, and zygotes expressing a non-phosphorylatable Ste5 mutant often lyse at this stage, presumably because persistent pheromone signaling maintains polarized growth despite mechanical pressure. Thus, in addition to extrinsic mechanical stress, intrinsic physical signals are sensed by similar mechanisms to orchestrate the different processes during yeast mating.
Figure 3. Cross talk between the CWI and the pheromone response pathway upon intrinsic cell wall stress.
Osmotic stress prevents pheromone signaling independent of Ste5 membrane-recruitment
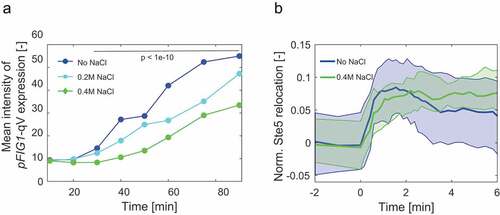
Several studies investigated crosstalk between the pheromone response- and the high osmolarity HOG pathway [Citation27,Citation33,Citation34]. Experiments using fluorescent reporters to monitor the transcriptional output indicated that cells are not able to simultaneously activate both pathways when exposed to both stimuli at the same time. Further analysis revealed that insulation is mainly achieved by the respective scaffolds and MAP kinases. Indeed, a Fus3 mutant that is not inhibited when cells are exposed to pheromone and osmotic stress at the same time, leads to simultaneous activation of both pathways [Citation35]. Curiously, these cells were more sensitive to low pheromone concentrations. Consistent with this notion, a phospho-proteomic study assessing the presence of doubly-phosphorylated, active Fus3 and Hog1 showed that various co-stimulation regimes result in rapid but transient Hog1 activation and downregulation of Fus3 [Citation36]. In contrast, another study observed transcriptional readouts for both pathways after co-stimulation for two hours. However, since cells exposed to high osmolarity adapt by increasing intracellular glycerol to equilibrate the turgor pressure, it is expected that Fus3 increases as soon as Hog1 activity ceases. To corroborate these findings, we measured pFIG1-qV induction as a transcriptional readout of the mating pheromone pathway at different time points after NaCl addition [Citation10,Citation37,Citation38]. Indeed, pheromone signaling was dampened in cells exposed to different concentrations of NaCl (), suggesting that activation of the pheromone response pathway is inhibited in cells exposed to high osmolarity. Surprisingly, however, the recruitment of Ste5 to the membrane upon exposure of cells to alpha factor was not affected in the presence of high osmolarity () [Citation10]. Thus, in contrast to crosstalk imposed by the cell cycle and mechanical stress, Hog1 activation by high osmolarity blocks pheromone signaling downstream of Ste5. The critical Hog1 substrate(s) mediating this crosstalk remains unclear, but the rapid kinetics suggest that Hog1 likely phosphorylates and thereby inactivates one or several of the critical components of the pheromone signaling pathway [Citation39]. Since Ste5 is a major regulatory hub and phosphorylated at over 40 unique sites, we speculate that Hog1-dependent phosphorylation may interfere with its signaling capacity beyond membrane recruitment.
Figure 4. Crosstalk between the HOG and the pheromone response pathways is independent of Ste5 recruitment to the cell membrane.
Outlook
Genetic and biochemical studies not only unraveled the components and feedback controls of individual MAP kinase pathways, but also identified the mechanisms that link them to adjust cellular responses to diverse extra- and intracellular conditions. Thanks to these crosstalk mechanisms the otherwise insulated pathways function as an interconnected network with different outputs. Scaffold proteins are known to provide pathway specificity in space and time, but also emerge as regulatory nodes for crosstalk regulation. For example, membrane recruitment of Ste5 is regulated by the cell cycle and mechanical stress, thereby preventing pheromone signaling during adverse conditions. However, additional MAP kinase-dependent crosstalk mechanisms exist and it will be important to identify their relevant substrates. Moreover, other intrinsic or external stress conditions affect MAP-kinase signaling and thus further work will be required to understand the complex wiring and outputs of MAP-kinase networks.
Acknowledgments
We thank J. Tilma and I. Stoffel for technical assistance, A. Smith for critical reading of the manuscript and Peter laboratory for helpful discussions. Work in the Peter laboratory is supported by an ERC award, a SNF project grant, ETH Zurich and the Global Research Laboratory (NRF-2015K1A1A2033054) through the National Research Foundation of Korea (NRF). The authors declare no competing financial interests.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Engelberg D, Perlman R, Levitzki A. Transmembrane signaling in Saccharomyces cerevisiae as a model for signaling in metazoans: state of the art after 25 years. Cell Signal. 2014;26:2865–2878.
- Qi M, Elion EA. MAP kinase pathways. J Cell Sci. 2005;118:3569–3572.
- Brewster JL, Gustin MC. Hog1: 20 years of discovery and impact. Sci Signal. 2014;7:re7.
- Cullen PJ, Sprague GF Jr. The regulation of filamentous growth in yeast. Genetics. 2012;190:23–49.
- Lee J, Liu L, Levin DE. Stressing out or stressing in: intracellular pathways for SAPK activation. Curr Genet. 2019;65:417–421.
- Brent R. Cell signaling: what is the signal and what information does it carry? FEBS Lett. 2009;583:4019–4024.
- Neiman AM. Sporulation in the budding yeast Saccharomyces cerevisiae. Genetics. 2011;189:737–765.
- Delarue M, Poterewicz G, Hoxha O, et al. SCWISh network is essential for survival under mechanical pressure. Proc Natl Acad Sci U S A. 2017;114:13465–13470.
- Mishra R, van Drogen F, Dechant R, et al. Protein kinase C and calcineurin cooperatively mediate cell survival under compressive mechanical stress. Proc Natl Acad Sci U S A. 2017;114:13471–13476.
- van Drogen F, Mishra R, Rudolf F, et al. Mechanical stress impairs pheromone signaling via Pkc1-mediated regulation of the MAPK scaffold Ste5. J Cell Biol. 2019;218:3117–3133.
- Dohlman HG, Slessareva JE. Pheromone signaling pathways in yeast. Sci STKE. 2006;2006:cm6.
- Butty AC, Pryciak PM, Huang LS, et al. The role of Far1p in linking the heterotrimeric G protein to polarity establishment proteins during yeast mating. Science. 1998;282:1511–1516.
- Johnson DI, Pringle JR. Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J Cell Biol. 1990;111:143–152.
- Moskow JJ, Gladfelter AS, Lamson RE, et al. Role of Cdc42p in pheromone-stimulated signal transduction in Saccharomyces cerevisiae. Mol Cell Biol. 2000;20:7559–7571.
- Elion EA. Pheromone response, mating and cell biology. Curr Opin Microbiol. 2000;3:573–581.
- Ekiel I, Sulea T, Jansen G, et al. Binding the atypical RA domain of Ste50p to the unfolded Opy2p cytoplasmic tail is essential for the high-osmolarity glycerol pathway. Mol Biol Cell. 2009;20:5117–5126.
- Tanaka K, Tatebayashi K, Nishimura A, et al. Yeast osmosensors Hkr1 and Msb2 activate the Hog1 MAPK cascade by different mechanisms. Sci Signal. 2014;7:ra21.
- Posas F, Wurgler-Murphy SM, Maeda T, et al. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 1996;86:865–875.
- Zarzov P, Mazzoni C, Mann C. The SLT2(MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. Embo J. 1996;15:83–91.
- van Drogen F, Peter M. Spa2p functions as a scaffold-like protein to recruit the Mpk1p MAP kinase module to sites of polarized growth. Curr Biol. 2002;12:1698–1703.
- Stevenson BJ, Rhodes N, Errede B, et al. Constitutive mutants of the protein kinase STE11 activate the yeast pheromone response pathway in the absence of the G protein. Genes Dev. 1992;6:1293–1304.
- van Drogen F, O’Rourke SM, Stucke VM, et al. Phosphorylation of the MEKK Ste11p by the PAK-like kinase Ste20p is required for MAP kinase signaling in vivo. Curr Biol. 2000;10:630–639.
- Esch RK, Errede B. Pheromone induction promotes Ste11 degradation through a MAPK feedback and ubiquitin-dependent mechanism. Proc Natl Acad Sci U S A. 2002;99:9160–9165.
- Inouye C, Dhillon N, Thorner J. Ste5 RING-H2 domain: role in Ste4-promoted oligomerization for yeast pheromone signaling. Science. 1997b;278:103–106.
- Winters MJ, Lamson RE, Nakanishi H, et al. A membrane binding domain in the ste5 scaffold synergizes with gbetagamma binding to control localization and signaling in pheromone response. Mol Cell. 2005;20:21–32.
- Inouye C, Dhillon N, Durfee T, et al. Mutational analysis of STE5 in the yeast Saccharomyces cerevisiae: application of a differential interaction trap assay for examining protein-protein interactions. Genetics. 1997a;147:479–492.
- O’Rourke SM, Herskowitz I. The Hog1 MAPK prevents cross talk between the HOG and pheromone response MAPK pathways in Saccharomyces cerevisiae. Genes Dev. 1998;12:2874–2886.
- Strickfaden SC, Winters MJ, Ben-Ari G, et al. A mechanism for cell-cycle regulation of MAP kinase signaling in a yeast differentiation pathway. Cell. 2007;128:519–531.
- Repetto MV, Winters MJ, Bush A, et al. CDK and MAPK synergistically regulate signaling dynamics via a shared multi-site phosphorylation region on the scaffold protein Ste5. Mol Cell. 2018;69:938–952 e936.
- Cullen PJ, Sabbagh W Jr., Graham E, et al. A signaling mucin at the head of the Cdc42- and MAPK-dependent filamentous growth pathway in yeast. Genes Dev. 2004;18:1695–1708.
- O’Rourke SM, Herskowitz I. A third osmosensing branch in Saccharomyces cerevisiae requires the Msb2 protein and functions in parallel with the Sho1 branch. Mol Cell Biol. 2002;22:4739–4749.
- Davi V, Tanimoto H, Ershov D, et al. Mechanosensation dynamically coordinates polar growth and cell wall assembly to promote cell survival. Dev Cell. 2018;45:170–182 e177.
- McClean MN, Mody A, Broach JR, et al. Cross-talk and decision making in MAP kinase pathways. Nat Genet. 2007;39:409–414.
- Patterson JC, Klimenko ES, Thorner J. Single-cell analysis reveals that insulation maintains signaling specificity between two yeast MAPK pathways with common components. Sci Signal. 2010;3:ra75.
- Hall JP, Cherkasova V, Elion E, et al. The osmoregulatory pathway represses mating pathway activity in Saccharomyces cerevisiae: isolation of a FUS3 mutant that is insensitive to the repression mechanism. Mol Cell Biol. 1996;16:6715–6723.
- Vaga S, Bernardo-Faura M, Cokelaer T, et al. Phosphoproteomic analyses reveal novel cross-modulation mechanisms between two signaling pathways in yeast. Mol Syst Biol. 2014;10:767.
- Lee B, Jeong SG, Jin SH, et al. Submitted. Quantitative analysis of yeast MAPK signaling networks and crosstalk using a microfluidic device.
- Pelet S. Nuclear relocation of Kss1 contributes to the specificity of the mating response. Sci Rep. 2017;7:43636.
- Nagiec MJ, Dohlman HG. Checkpoints in a yeast differentiation pathway coordinate signaling during hyperosmotic stress. PLoS Genet. 2012;8:e1002437.
