ABSTRACT
G1 cell cycle progression is controlled largely by growth factors in early G1 indicating that it is appropriate to divide and by nutrients in late G1 indicating sufficient raw material for cell division. We previously mapped a late G1 cell cycle checkpoint for lipids upstream from a mammalian target of rapamycin complex 1 (mTORC1)-mediated checkpoint and downstream from a mid-G1 checkpoint known as the Restriction point. We therefore investigated a role for lipids in progression through late G1 into S-phase. Quiescent BJ-hTERT human fibroblasts were primed with 10% fetal bovine serum (FBS) for 3.5 h at which time, cells were treated with a mixture of lipids and carrier bovine serum albumin (BSA) along with [3 H]-thymidine deoxyribose ([3 H]-TdR) to monitor progression into S-phase. Surprisingly, BSA by itself was more effective than FBS in promoting progression to S-phase – the lipids had no impact on progression. While insulin strongly stimulated mTORC1 activity, it did not impact on [3 H]-TdR incorporation. Although BSA modestly elevated mTORC1 activity, rapamycin strongly inhibited BSA-induced progression to S-phase. BSA treatment promoted mitosis, but not progression through a second G1. Thus, after priming quiescent cells with FBS, albumin was sufficient to promote progression into S-phase. The BSA was not simply a source of amino acids in that amino acids were present in the culture media. We propose that the presence of albumin – the most abundant protein in serum – reflects a broader availability of essential amino acids needed for cell growth.
Introduction
The decision by cells whether to divide or not to divide is largely determined during G1 of the cell cycle [Citation1]. Consistent with the importance of G1 regulation, most significant driver mutations in cancer are in genes that regulate passage through G1 [Citation2]. G1 can be divided into two distinct regions – early and late that are separated by a growth-factor-dependent Restriction point [Citation3,Citation4]. Growth factor signals are needed to get past the Restriction point and this regulatory site is where signals are informing the cell that it is appropriate to divide [Citation1]. If there are no growth factors present, cells exit the cycle and enter a state of quiescence commonly referred to as G0 [Citation1]. Passage through late G1 is largely governed by determining whether there are sufficient raw materials for getting the job done. There are several metabolic checkpoints in late G1 that check for both nutrient sufficiency and genomic integrity [Citation1,Citation2]. The metabolic checkpoints monitor the presence of essential amino acids, glutamine, and lipids [Citation5,Citation6]. There is also a checkpoint mediated by mammalian target of rapamycin complex 1 (mTORC1) [Citation6,Citation7]. This site, which has also been referred to as the Restriction point [Citation8], requires active mTORC1, that suppresses TGFβ signals that arrest cells in late G1 [Citation9]. Cell cycle arrest by rapamycin requires activation of TGFβ signals and suppression of Rb phosphorylation [Citation9]. Growth factor signals between mitosis and the original Restriction point – about 3.5 h [Citation10] – prevent exit to G0 and at this point growth factor signals are no longer needed until after mitosis [Citation4,Citation10]. While quiescent G0 cells can be restarted by addition of growth factors, additional growth factors that activate mTORC1 have also been reported for passage through the late G1 checkpoint mediated by mTORC1 [Citation1].
Pledger and Stiles several years ago described a commitment and progression model for cell progression from quiescence/G0 into S-phase and mitosis [Citation11,Citation12]. In these early studies, quiescent fibroblasts could be primed by a “competence” factor such platelet-derived growth factor for as little as 1 h followed by treatment with platelet-poor-plasma [Citation11] or with plasma from hypophysectomized rats deficient in progression factors and IGF1, which did promote progression to S-phase [Citation12]. Thus, while IGF1 helped promote the progression of primed fibroblasts through late G1 there were clearly other factor(s) in the platelet-poor-plasma and the plasma from hypophysectomized rats critical for G1 → S-phase progression.
We previously identified a late G1 cell cycle checkpoint for lipids upstream from a mTORC1-mediated checkpoint and downstream from the mid-G1 Restriction point [Citation5]. We wanted to investigate a role for lipids in progression through late G1 into S-phase. To this end, we examined whether a mixture of lipids could act as a progression factor and promote the progression of primed human fibroblasts into S-phase. The mixture of lipids was combined with bovine serum albumin (BSA) as carrier protein for the lipids. What we found was very surprising – the BSA, by itself, potently promoted progression of primed human fibroblasts through late G1 into S-phase and the presence of lipids with the BSA had no additive effect. While the BSA-induced cell cycle progression was highly sensitive to rapamycin, BSA had a very modest effect on mTORC1 activity. In contrast, IGF1 strongly increased mTORC1 activity, but had no impact of G1 → S-phase progression. We are proposing that BSA represents an abundance of nutrients that informs the cell that progression into S-phase and replicating the genome is feasible.
Materials and methods
Cells and cell culture conditions and cell viability
The BJ-hTERT cell line used in this study was obtained from American Type Culture Collection. No authentication was performed by the authors. Cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma) supplemented with 10% fetal bovine serum (FBS) (Sigma). Cell viability was determined by counting the number of adherent cells remaining after treatment.
Materials
Reagents were obtained from the following sources. Antibodies against S6 K (9202), P-S6 K T389 (9234), P-AKT S473 (9272), and GAPDH (5174) were obtained from Cell Signaling Technology; antimouse- and anti-rabbit HRP-conjugated secondary antibodies were obtained from Proteintech. Fatty acid mixture (11905) was obtained from Invitrogen, and fatty-acid-free bovine serum albumin (A9205) was obtained from Sigma. Rapamycin (R-5000) was obtained from LC Laboratories. Ultima Gold scintillation fluid (6013681) and [3H]-thymidine deoxyribose ([3 H]-TdR) (20 Ci/mmol, 1 mCi/ml) (NET-027E) were obtained from PerkinElmer. DMEM (D6429) high glucose, MEM (M4655) and MEM Amino acids (50x) solution (M5550) was obtained from Sigma. Insulin (I9278), EIPA and Human Globulin fraction (G3637) were obtained from Sigma.
Lipid mix supplementation
A fatty acid mix was obtained from Invitrogen (11905) and supplied to cells as a 1:200 dilution complexed with 10% BSA (Sigma) in a 2:1 ratio for the final concentration of lipids in the medium of 0.375 mg/liter. The composition of the fatty acid mixture is provided in supplemental Table S1.
Thymidine incorporation assay
Cells were labeled with 1 Ci/ml [3H]-TdR. At the indicated times, cells were washed twice with 1 ml of phosphate-buffered saline, and then precipitated twice with 1 ml of 10% trichloroacetic acid. The precipitates were solubilized in 0.5 ml of 0.5% SDS, 0.5 M NaOH solution, and the extent of [3 H]-TdR incorporation was quantified using 75 ul of sample and 3 ml of scintillation fluid.
Western blot analysis
Proteins were extracted from cultured cells in M-PER (Thermo Scientific 78501). Equal amounts of proteins were subjected to SDS-PAGE on polyacrylamide separating gels. Electrophoresed proteins were then transferred to nitrocellulose membrane. After transfer, membranes were blocked in an isotonic solution containing 5% nonfat dry milk in phosphate-buffered saline. Membranes were then incubated with primary antibodies as described in the text. The dilutions were used as per vendor’s instructions. Depending on the origin of the primary antibody, either anti-mouse- or anti-rabbit HRP-conjugated IgG was used for detection using the ECL system (Kindle Biosciences 1002).
Statistical analysis
Statistical significance was analyzed using Graph Pad Prism 8 software. Comparisons between two sets of samples were made using unpaired t-tests. For multiple comparisons, an unpaired one-way analysis of variance (ANOVA) was performed. Data are plotted as the mean with standard deviation. The p-values are as follows: *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001. Not significant (ns) means p > 0.05.
Results
BSA stimulates the progression of fetal bovine serum (FBS)-primed BJ-hTERT human fibroblasts from G0 into S-phase
In the absence of serum growth factors, cycling non-transformed cells exit the cycle and enter a state of quiescence referred to as G0. Restoring serum growth factors can stimulate quiescent cells to reenter the cell cycle and proceed to S-phase where the genome is replicated and then to mitosis [Citation6]. As discussed in the Introduction, the process of reentry and progression can be broken up into two distinct stages – commitment and progression [Citation11,Citation12]. Commitment could be achieved with as little a 1 h treatment with platelet-derived growth factor, whereas progression was more complicated involving IGF1 and other undetermined factor(s) in serum [Citation11,Citation12]. We recently identified a late G1 cell cycle checkpoint that monitored the presence of fatty acids (FAs) [Citation5]. We therefore wanted to determine whether lipids in serum contribute to late G1 cell cycle progression into S-phase. We used the human fibroblast cell line BJ-hTERT, which arrests very well in G0 [Citation6]. BJ-hTERT cells were put in serum-free medium for 48 h, at which time all cells were treated with 10% FBS for 3.5 h to prime the BJ-hTERT cells. At this point the cells were washed and then treated with medium containing [3 H]-TdR without FBS (-FBS) and with FBS (+FBS) for 24 h. As shown in (columns 1 and 2), FBS-primed cells shifted to medium lacking FBS incorporated approximately 20% as much [3 H]-TdR as the cells that were put back into medium containing 10% FBS. This result demonstrates that the 3.5 h treatment was not sufficient to promote progression to S-phase where [3 H]-TdR gets incorporated into DNA. To determine whether FAs could promote the progression of FBS-primed BJ-hTERT cells into S-phase we added a mixture of FAs along with fatty acid-free BSA carrier protein for the lipids (FA + BSA). We also added the FAs (FA) and BSA (BSA) separately ( (columns 3–5)). These data were very surprising in that 0.05% BSA stimulated [3H]-TdR incorporation to the same extent as FBS. The FAs by themselves had little or no effect on [3H]-TdR incorporation, nor did it enhance the impact of the BSA on [3H]-TdR incorporation. We next performed a dose curve for the ability of BSA to induce [3H]-TdR incorporation in FBS-primed BJ-hTERT cells from 0.5% to the physiological concentration of albumin in circulating plasma of 3% [Citation13]. As shown in , when the BSA concentration reached 3%, there was a fourfold greater [3H]-TdR incorporation than in the BJ-hTERT cells primed with and treated with FBS. These data surprisingly demonstrate that quiescent fibroblasts primed for 3.5 h with FBS can be induced to progress into S-phase more effectively by BSA than by treating with FBS for the entire period of observation. Importantly, progression into S-phase was accomplished in the absence of growth factors.
Figure 1. BSA stimulates the progression of FBS-primed BJ-hTERT human fibroblasts from G0 into S-phase. (a) BJ-hTERT cells were plated at 30% confluency in DMEM 10% FBS. After 24 h, cells were shifted to DMEM 0% FBS for 48 h. After this, media was replaced with DMEM 10% FBS for 3.5 h. This media was washed off and replaced with DMEM (-FBS), DMEM 10% FBS (+FBS), DMEM + fatty acid mix/fatty acid-free BSA (FA + 0.05% BSA), DMEM 0.05% BSA (0.05% BSA), and DMEM fatty acid mix (FA) for 24 h. Cells were also labeled with [3H]-TdR for the final 24 h of treatment, after which they were collected and the incorporated label [3H]-TdR was determined by scintillation counting. Relative [3H]-TdR incorporation is represented as counts per minute and normalized to the positive control +FBS which has been given a value of 100%. Significance asterisks are compared with the control. The data is represented as mean ± SD and is the result of at least three independent experiments each consisting of two replicates per condition. (b) Cells were plated, starved of FBS, primed with +FBS media for 3.5 h as explained in (a) before replacement of media with +FBS or indicated concentrations of BSA for 24 h. Cells were also labeled with [3H]-TdR for the final 24 h of treatment, after which they were collected and the incorporated [3H]-TdR was determined by scintillation counting. Relative [3H]-TdR incorporation is represented as counts per minute and normalized to the positive control +FBS which has been given a value of 100%. **, p ≤ 0.01, ****, p ≤ 0.0001. Not significant (ns) means p > 0.05.
![Figure 1. BSA stimulates the progression of FBS-primed BJ-hTERT human fibroblasts from G0 into S-phase. (a) BJ-hTERT cells were plated at 30% confluency in DMEM 10% FBS. After 24 h, cells were shifted to DMEM 0% FBS for 48 h. After this, media was replaced with DMEM 10% FBS for 3.5 h. This media was washed off and replaced with DMEM (-FBS), DMEM 10% FBS (+FBS), DMEM + fatty acid mix/fatty acid-free BSA (FA + 0.05% BSA), DMEM 0.05% BSA (0.05% BSA), and DMEM fatty acid mix (FA) for 24 h. Cells were also labeled with [3H]-TdR for the final 24 h of treatment, after which they were collected and the incorporated label [3H]-TdR was determined by scintillation counting. Relative [3H]-TdR incorporation is represented as counts per minute and normalized to the positive control +FBS which has been given a value of 100%. Significance asterisks are compared with the control. The data is represented as mean ± SD and is the result of at least three independent experiments each consisting of two replicates per condition. (b) Cells were plated, starved of FBS, primed with +FBS media for 3.5 h as explained in (a) before replacement of media with +FBS or indicated concentrations of BSA for 24 h. Cells were also labeled with [3H]-TdR for the final 24 h of treatment, after which they were collected and the incorporated [3H]-TdR was determined by scintillation counting. Relative [3H]-TdR incorporation is represented as counts per minute and normalized to the positive control +FBS which has been given a value of 100%. **, p ≤ 0.01, ****, p ≤ 0.0001. Not significant (ns) means p > 0.05.](/cms/asset/ff193408-9b08-4b93-9f2c-a93903555015/kccy_a_1795999_f0001_b.gif)
Suppression of macropinocytosis inhibits progression into S-phase by both FBS and BSA
The uptake of protein into cells requires macropinocytosis [Citation14]. To validate that the BSA-induced progression into S-phase required macropinocytosis we used 5-(N-ethyl-N-isopropyl) amiloride (EIPA), which blocks macropinocytosis [Citation14,Citation15]. As shown in , EIPA blocked progression into S-phase by both BSA and FBS. These data suggest not only that BSA requires macropinocytosis, but that FBS-induced S-phase progression similarly requires uptake of protein. To demonstrate that the result is not simply an artifact of EIPA treatment, we examined the impact of EIPA on the phosphorylation of AKT at the mTORC2 site at S473, which is very growth factor responsive (Toschi et al., 2009). As shown in , EIPA had no impact on the levels of AKT phosphorylation in cells treated with either BSA or FBS. These data demonstrate that EIPA is not just interfering with the transduction of intracellular signals. The data also suggest the macropinocytosis of proteins contained in FBS is critical for S-phase progression induced by serum.
Figure 2. Suppression of macropinocytosis blocks albumin-induced cell cycle progression. (a) BJ-hTERT cells were plated at 30% confluency in DMEM 10% FBS. After 24 h, cells were shifted to DMEM 0% FBS for 48 h. After this, media was replaced with DMEM 10% FBS for 3.5 h. This media was washed off and cells were treated with media conditions +FBS, +FBS + EIPA 75uM, 3% BSA, and 3% BSA + EIPA 75uM for 24 h. Each final 24 h condition also contained [3H]-TdR. After 24 h, cells were collected and the incorporated [3 H]-TdR was determined by scintillation counting. (b) Cells were plated and treated as in (a) after which cells were harvested and the levels of Akt phosphorylation at Ser473 was determined by Western blot analysis. (c) BJ-hTERT cells were plated, starved of FBS, primed with +FBS media for 3.5 h as explained in (a) before replacement with media conditions -FBS, +FBS, 3% bovine serum globulin fraction. Each final 24 h condition also contained [3 H]-TdR. After 24 h, cells were collected and the incorporated label ([3 H]-TdR) was determined by scintillation counting. In both (a) and (c) relative [3 H]-TdR incorporation is represented as counts per minute and normalized to the positive control +FBS which has been given a value of 100%. Significance asterisks are compared with the control unless otherwise noted with brackets. The data is represented as mean ± SD and is the result of at least three independent experiments each consisting of two replicates per condition. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001. Not significant (ns) means p > 0.05.
![Figure 2. Suppression of macropinocytosis blocks albumin-induced cell cycle progression. (a) BJ-hTERT cells were plated at 30% confluency in DMEM 10% FBS. After 24 h, cells were shifted to DMEM 0% FBS for 48 h. After this, media was replaced with DMEM 10% FBS for 3.5 h. This media was washed off and cells were treated with media conditions +FBS, +FBS + EIPA 75uM, 3% BSA, and 3% BSA + EIPA 75uM for 24 h. Each final 24 h condition also contained [3H]-TdR. After 24 h, cells were collected and the incorporated [3 H]-TdR was determined by scintillation counting. (b) Cells were plated and treated as in (a) after which cells were harvested and the levels of Akt phosphorylation at Ser473 was determined by Western blot analysis. (c) BJ-hTERT cells were plated, starved of FBS, primed with +FBS media for 3.5 h as explained in (a) before replacement with media conditions -FBS, +FBS, 3% bovine serum globulin fraction. Each final 24 h condition also contained [3 H]-TdR. After 24 h, cells were collected and the incorporated label ([3 H]-TdR) was determined by scintillation counting. In both (a) and (c) relative [3 H]-TdR incorporation is represented as counts per minute and normalized to the positive control +FBS which has been given a value of 100%. Significance asterisks are compared with the control unless otherwise noted with brackets. The data is represented as mean ± SD and is the result of at least three independent experiments each consisting of two replicates per condition. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001. Not significant (ns) means p > 0.05.](/cms/asset/658fe64d-324e-4670-b3c8-c6796f6ea503/kccy_a_1795999_f0002_b.gif)
Since there are proteins other than albumin in FBS, we investigated whether other proteins could promote S-phase progression. We used human γ-globulin alongside FBS to promote progression of FBS-primed BJ-hTERT into S-phase. As shown in , 3% γ-globulin, like BSA, stimulated progression to S-phase more effectively than FBS. Thus, it appears that it is the protein in serum and BSA that is critical for promoting the S-phase progression of FBS-primed BJ-hTERT. Since albumin is the most abundant protein in serum [Citation13], it is likely that the key protein factor in serum is albumin.
The role of mTORC1 and insulin in the late G1 progression of FBS-primed BJ-hTERT cells
A key mediator of late G1 progression is mTORC1, which mediates a late G1 checkpoint that is close to the G1 → S-phase transition site [Citation6,Citation7,Citation9]. mTOR has been referred to as an integrator of growth factor and nutrient signals for cell cycle progression [Citation16–Citation18]. We therefore examined the effect of insulin, which is a strong inducer of mTORC1 [Citation19] on the S-phase progression of FBS-primed BJ-hTERT cells. As shown in , insulin was unable to increase [3H]-TdR incorporation in the FBS-primed BJ-hTERT cells. We also examined the effect of the mTORC1 inhibitor rapamycin on the ability of FBS and BSA to induce [3H]-TdR incorporation in FBS-primed BJ-hTERT cells. As shown in , rapamycin inhibited both FBS- and BSA-induced S-phase progression as indicated by [3H]-TdR incorporation. We next compared the effect of FBS, BSA and insulin on mTOR activity as indicated by phosphorylation of S6 K (mTORC1) and Akt (mTORC2). As with the [3 H]-TdR incorporation experiment, cells were starved of serum for 48 h, primed with FBS and then washed and treated with BSA, insulin, -FBS media or 10% FBS for 30 min. As shown in , removal of FBS and 30 min treatment with -FBS media resulted in a significant decrease in ribosomal subunit S6 kinase (S6 K) and Akt phosphorylation. Treatment with either FBS or Insulin sustained elevated levels of phosphorylated S6 K and phosphorylation of Akt at the mTORC2 site at Ser473. In contrast, removal of FBS and subsequent treatment with BSA resulted in weakly elevated phosphorylation of S6 K and Akt (). Rapamycin completely inhibited the phosphorylation of S6 K induced by FBS and BSA. The data in reveal that in spite of a strong activation of mTORC1, insulin by itself, could not induce progression of primed BJ-hTERT cells into S-phase. In contrast, the presence of BSA resulted in lower, but significant levels of mTORC1 activity while strongly promoting progression of primed BJ-hTERT cells into S-phase. Importantly, the induction of S-phase progression by BSA was suppressed by rapamycin. These data indicate that mTORC1 is necessary for S-phase progression in response to both FBS and BSA, but not sufficient as demonstrated by the strong induction of mTORC1 and the lack of induction of S-phase progression by insulin.
Figure 3. The role of mTORC1 and insulin in the late G1 progression of FBS-primed BJ-hTERT cells. (a) BJ-hTERT cells were plated at 30% confluency in DMEM 10% FBS. After 24 h, cells were shifted to DMEM 0% FBS for 48 h. After this, media was replaced with DMEM 10% FBS for 3.5 h. This media was washed off and cells were treated with conditions -FBS, +FBS, 3% BSA, DMEM + Insulin 10uM (Insulin), DMEM 10% FBS + Rapamycin 20uM (+FBS + Rapa) and DMEM 3% BSA + Rapamycin 20uM (BSA + Rapa) for 24 h. Each final 24 h condition also contained [3H]-TdR. After 24 h, cells were collected and the incorporated [3H]-TdR was determined by scintillation counting. Relative [3H]-TdR incorporation is represented as counts per minute and normalized to the positive control +FBS which has been given a value of 100%. Significance asterisks are compared with the control unless otherwise denoted with additional brackets. The data is represented as mean ± SD and is the result of at least three independent experiments each consisting of two replicates per condition. (b) As described in (a), cells were plated, deprived of FBS for 48 h then primed with +FBS for 3.5 h. After this, media was replaced with conditions indicated for 30 min. After 30 min, cells were harvested and protein levels determined by Western blot analysis. *, p ≤ 0.05, ****, p ≤ 0.0001. Not significant (ns) means p > 0.05.
![Figure 3. The role of mTORC1 and insulin in the late G1 progression of FBS-primed BJ-hTERT cells. (a) BJ-hTERT cells were plated at 30% confluency in DMEM 10% FBS. After 24 h, cells were shifted to DMEM 0% FBS for 48 h. After this, media was replaced with DMEM 10% FBS for 3.5 h. This media was washed off and cells were treated with conditions -FBS, +FBS, 3% BSA, DMEM + Insulin 10uM (Insulin), DMEM 10% FBS + Rapamycin 20uM (+FBS + Rapa) and DMEM 3% BSA + Rapamycin 20uM (BSA + Rapa) for 24 h. Each final 24 h condition also contained [3H]-TdR. After 24 h, cells were collected and the incorporated [3H]-TdR was determined by scintillation counting. Relative [3H]-TdR incorporation is represented as counts per minute and normalized to the positive control +FBS which has been given a value of 100%. Significance asterisks are compared with the control unless otherwise denoted with additional brackets. The data is represented as mean ± SD and is the result of at least three independent experiments each consisting of two replicates per condition. (b) As described in (a), cells were plated, deprived of FBS for 48 h then primed with +FBS for 3.5 h. After this, media was replaced with conditions indicated for 30 min. After 30 min, cells were harvested and protein levels determined by Western blot analysis. *, p ≤ 0.05, ****, p ≤ 0.0001. Not significant (ns) means p > 0.05.](/cms/asset/f1fd7d88-abf2-4e1d-bf23-960e13a7b032/kccy_a_1795999_f0003_b.gif)
BSA treatment allows for one cell division of the BJ-hTERT cells followed by cell death
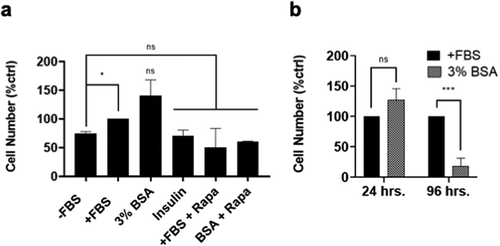
The data in and demonstrate that BSA and perhaps other proteins contained in serum are driving FBS-primed BJ-hTERT fibroblasts into S-phase. We wished to determine whether these cells actually undergo mitosis and dividing. The FBS-primed cells were treated with FBS, BSA and insulin for 24 h – at which time the number of viable cells remaining on plates was determined. As shown in there was an increase in cell number in the cells treated with FBS and BSA with the BSA-treated cells having significantly more cells than the FBS-treated cells. There was no increase in cell number seen in cells treated with insulin nor was there elevated numbers in the FBS- and BSA-treated cells if rapamycin was included (). However, if the cells were maintained in BSA for 4 days, the cells were mostly dead (). Thus, while the cells were able to undergo one cell division, they apparently did not exit into G0 at the Restriction point and this ultimately led to cell death.
Figure 4. BSA cannot sustain cell cycle progression long term. (a) BJ-hTERT cells were plated at 30% confluency in DMEM 10% FBS. After 24 h, cells were shifted to DMEM 0% FBS for 48 h. After this, media was replaced with DMEM 10% FBS for 3.5 h. This media was washed off and cells were treated with media conditions -FBS, +FBS, 3% BSA, DMEM + Insulin 10uM (Insulin), DMEM 10% FBS + Rapamycin 20uM (+FBS + Rapa) and DMEM 3% BSA + Rapamycin 20uM (BSA + Rapa) for 24 h. After 24 h, viable adherent cells were collected and counted with a hemocytometer. (b) Cells were plated and treated as described in (a). After 24 and 96 h, viable (attached) cells were collected and counted with a hemocytometer. In both (a) and (b) cell number is represented as percent of positive control +FBS which has been given a value of 100%. Significance asterisks are compared with the control unless otherwise denoted with additional brackets. The data is represented as mean ± SD and is the result of at least three independent experiments. *, p ≤ 0.05, ***, p ≤ 0.001. Not significant (ns) means p > 0.05.
Effect of amino acids on mTORC1 activity and cell cycle progression
Bar-Sagi and colleagues reported previously that macropinocytosis of protein is an amino acid supply route in Ras-transformed cells [Citation14]. In this study, the medium contained the standard amino acid levels of DMEM. Thus, it is unlikely that it was the amino acids generated from BSA that was driving the FBS-primed BJ-hTERT cells into S-phase. Amino acids are known to contribute to the activation of mTORC1, an important regulator of cell cycle progression from late G1 into S-phase [Citation6,Citation20,Citation21]. We therefore looked at the effect on mTORC1 activity of adding additional amino acids to FBS-primed BJ-hTERT cells. As shown in , the addition of amino acids actually did increase the levels of phosphorylated S6 K. However, amino acids had no impact on progression to S-phase as determined by incorporation of [3H]-TdR (). Thus, the generation of amino acids from BSA does not explain the ability of BSA to drive FBS-primed BJ-hTERT cells into S-phase.
Figure 5. Effect of BSA and amino acids on cell cycle progression and mTORC1 activity. (a) BJ-hTERT cells were plated at 30% confluency in MEM 10% FBS. After 24 h, cells were shifted to MEM 0% FBS for 48 h. After 48 h, media was replaced with MEM 10% FBS for 3.5 h. This media was washed off and cells were treated with media conditions -FBS, 3% BSA, and MEM with amino acid concentrations as indicated for 24 h. The next day cells were harvested and protein levels determined by Western blot analysis. (b) As described in (a), cells were plated, deprived of FBS for 48 h and then primed with +FBS for 3.5 h. This media was washed off and replaced with conditions indicated for 24 h. Each final 24 h condition also contained [3H]-TdR. After this 24 h treatment, cells were collected and the incorporated label ([3H]-TdR) was determined by scintillation counting. Relative [3H]-TdR incorporation is represented as counts per minute and normalized to the positive control MEM1X which has been given a value of 100%. ****, p ≤ 0.0001. Not significant (ns) means p > 0.05.
![Figure 5. Effect of BSA and amino acids on cell cycle progression and mTORC1 activity. (a) BJ-hTERT cells were plated at 30% confluency in MEM 10% FBS. After 24 h, cells were shifted to MEM 0% FBS for 48 h. After 48 h, media was replaced with MEM 10% FBS for 3.5 h. This media was washed off and cells were treated with media conditions -FBS, 3% BSA, and MEM with amino acid concentrations as indicated for 24 h. The next day cells were harvested and protein levels determined by Western blot analysis. (b) As described in (a), cells were plated, deprived of FBS for 48 h and then primed with +FBS for 3.5 h. This media was washed off and replaced with conditions indicated for 24 h. Each final 24 h condition also contained [3H]-TdR. After this 24 h treatment, cells were collected and the incorporated label ([3H]-TdR) was determined by scintillation counting. Relative [3H]-TdR incorporation is represented as counts per minute and normalized to the positive control MEM1X which has been given a value of 100%. ****, p ≤ 0.0001. Not significant (ns) means p > 0.05.](/cms/asset/2bc233e5-4ff0-43f1-9fc6-026d4fd6b967/kccy_a_1795999_f0005_b.gif)
Discussion
Based on a recent study from our lab where we identified a late G1 lipid checkpoint [Citation5], we began an investigation to determine whether lipids could drive cells into S-phase. From quiescence, 3.5 h of FBS treatment primes cells for progression, but cells do not go to S-phase and begin DNA synthesis. Surprisingly, we found that while lipids did not facilitate progression to S-phase, the albumin carrier protein promoted progression of FBS-primed BJ-hTERT fibroblasts into S-phase. Although albumin allowed for weakly sustained mTORC1 activity, the albumin-induced progression of FBS-primed BJ-hTERT cells into S-phase was inhibited by rapamycin – indicating that mTORC1 is required. Although insulin strongly induced mTORC1 activity, it was not sufficient to promote the progression of FBS-primed fibroblasts into S-phase. Insulin and IGF1 provide critical growth factor input in late G1 where both growth factor and nutrient signals are integrated by mTORC1 [Citation16,Citation17]. In this regard, the ability of FBS to promote the progression of FBS primed cells was blocked EIPA, which suppresses macropinocytosis [Citation14]. The EIPA treatment had no impact on the high levels of Akt phosphorylation, indicating that the ability to block S-phase progression was due to something in FBS that is brought into the cells by macropinocytosis – like albumin, which is the most abundant protein in serum [Citation13]. These data demonstrate that for cells in G0/quiescence, a 3.5 h treatment with FBS was all the growth factor treatment needed in that BSA was sufficient to promote progression to S-phase without growth factors present in the latter part of G1. The sensitivity to rapamycin indicated a growth factor-independent mTORC1 requirement.
The ability to promote the progression of FBS-primed BJ-hTERT cells into S-phase was not likely due to the generation of amino acids from the BSA as was reported for Ras-transformed cells [Citation14]. This is because standard concentrations of amino acids were present in the medium used for these studies. Progression into S-phase was also not restricted to albumin. γ-globulins could also promote S-phase progression of the FBS-primed BJ-hTERT cells. Thus, the effects reported here were apparently due to a high concentration of protein in the culture medium that gets taken up by macropinocytosis and is not dependent on individual amino acids. These results indicate that protein is providing something that amino acids cannot. We are proposing that while having adequate concentrations of amino acids present, it is difficult for the cell to determine whether there are adequate concentrations of all 20 amino acids. mTORC1 responds to some, but not all amino acids [Citation22]. However, the presence of a high concentration of protein in serum is a much broader indicator that there is an adequate supply of all 20 amino acids. The abundance of amino acids also ensures adequate precursors for nucleic acids and fatty acids. Since albumin is the most abundant protein in serum [Citation13], albumin is likely the most physiological relevant protein for facilitating progression from G1 into S-phase.
A recent report using cancer cells reported that in serum-starved HepG2/C3A hepatocellular carcinoma cells could be induced to progress from G1 into S-phase if treated with BSA [Citation13]. The major difference being that the cancer cells did not need priming with growth factors. Since the cells used in this study were cancer cells it is unclear whether these cells were exiting the cell cycle upon serum deprivation. However, cyclin D1 levels were not significantly reduced under serum-starved conditions suggesting that the cancer cells, as expected, were not going into quiescence where cyclin D1 levels are reduced in non-transformed cells [Citation6]. Thus, while the conditions for cell cycle progression from G1 into S-phase differ from conditions in our study, this work provides further evidence that albumin can promote G1 → S-phase cell cycle progression in the absence of growth factors. While it remains unclear as to the exact mechanism for albumin-driven cell cycle progression into S-phase, the sensitivity to EIPA implicates macropinocytosis and vesicle trafficking of albumin to the lysosome. The lysosome is also where mTORC1 functions as a nutrient sensor for both amino acids [Citation23] and lipids [Citation24,Citation25]. Consistent with the convergence of both albumin and mTORC1 on the lysosome and the sensitivity of albumin-induced cell cycle progression to rapamycin, it is likely that the lysosome plays a role in albumin-induced progression of FBS-primed BJ-hTERT fibroblasts into S-phase.
Supplemental Material
Download MS Word (16.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Foster DA, Yellen P, Xu L, et al. Regulation of G1 cell cycle progression: distinguishing the restriction point from a nutrient-sensing cell growth checkpoint(s). Genes Cancer. 2010;1:1124–1131.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674.
- Pardee AB. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974;71:1286–1290.
- Zetterberg A, Larsson O, Wiman KG. What is the restriction point? Curr Opin Cell Biol. 1995;7:835–842.
- Patel D, Salloum D, Saqcena M. A late G1 lipid checkpoint that is dysregulated in clear cell renal carcinoma cells. J Biol Chem. 2017;292:936–944.
- Saqcena M, Menon D, Patel D, et al. Amino acids and mTOR mediate distinct metabolic checkpoints in mammalian G1 cell cycle. PLoS One. 2013;8:e74157.
- Fingar DC, Richardson CJ, Tee AR, et al. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–216.
- Planas-Silva MD, Weinberg RA. The restriction point and control of cell proliferation. Curr Opin Cell Biol. 1997;9:768–772.
- Chatterjee A, Mukhopadhyay S, Tung K, et al. Rapamycin-induced G1 cell cycle arrest employs both TGF-β and Rb pathways. Cancer Lett. 2015;360:134–140.
- Zetterberg A, Larsson O. Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985;82:5365–5369.
- Pledger WJ, Stiles CD, Antoniades HN, et al. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977;74:4481–4485.
- Stiles CD, Capone GT, Scher CD, et al. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979;76:1279–1283.
- Ibrahim B, Stange J, Dominik A, et al. Albumin promotes proliferation of G1 arrested serum starved hepatocellular carcinoma cells. PeerJ. 2020;8:e8568.
- Commisso C, Davidson SM, Soydaner-Azeloglu RD, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637.
- Salloum D, Mukhopadhyay S, Tung K, et al. Mutant ras elevates dependence on serum lipids and creates a synthetic lethality for rapamycin. Mol Cancer Ther. 2014;13:733–741.
- Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol. 2013;15:555–564.
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171.
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35.
- Yoon MS. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients. 2017;9:1176.
- Avruch J, Long X, Ortiz-Vega S, et al. Amino acid regulation of TOR complex 1. Am J Phys Endocrin Metab. 2009;296:E592–602.
- Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med. 2012;18:524–533.
- Wolfson RL, Sabatini DM. The dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab. 2017;26:301–309.
- Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015;517:302–310.
- Frias MA, Mukhopadhyay S, Lehman E, et al. Phosphatidic acid drives mTORC1 lysosomal translocation in the absence of amino acids. J Biol Chem. 2020;295:263–274.
- Menon D, Salloum D, Bernfeld E, et al. Lipid sensing by mTOR complexes via de novo synthesis of phosphatidic acid. J Biol Chem. 2017;292:6303–6311.
