ABSTRACT
Myocardial infarction (MI) is the main cause of morbidity and mortality. Reperfusion ways can cause damage to cardiomyocytes. CircMAT2B, a novel circRNA, takes positive roles in regulating glucose metabolism under hypoxia. Therefore, we aimed to explore the effects of circMAT2B on MI. Oxygen-glucose deprivation (OGD)-induced H9c2 cell model was employed to stimulate MI. Ex-circMAT2B, si-circMAT2B, miR-133 inhibitor and relative control were transfected into H9c2 cells. qRT-PCR was employed to examine levels of circMAT2B and miR-133. Cell activity, apoptosis, ROS generation and release of inflammatory factors were assessed by CCK-8, flow cytometry, ROS species assay kit and ELISA, respectively. Moreover, the expression of apoptosis-related and pathway-related factors was detected through western blot analysis. The results showed that circMAT2B expression was notably up-regulated by OGD treatment. Moreover, circMAT2B knockdown could effectively decrease OGD-induced the increasing of apoptosis, ROS generation and the expression of IL-1β, IL-6 and TNF-α. Besides, miR-133 was positively regulated by si-circMAT2B. CircMAT2B knockdown attenuated OGD-induced H9c2 cell damage and alleviated OGD-induced the inhibition of PI3K/AKT and Raf/MEK/ERK pathways through up-regulating miR-133. In brief, circMAT2B knockdown works as an inflammatory inhibitor in OGD-induced H9c2 cells inflammatory injury through up-regulating miR-133.
Introduction
Myocardial ischemia (MI) happens when blood perfusion of the heart is decreased, causing a decrease in heart’s oxygen supply and abnormal myocardial energy metabolism [Citation1]. Long-term ischemia causes MI, which is the main cause of heart failure [Citation2,Citation3]. The common symptoms of MI are chest pain and angina with no prior warning signs [Citation4]. Myocardial hypoxia switches cellular metabolism to anaerobic respiration, with lactic acid accumulation, ATP depletion, overload of Na+ and Ca2+ and the suppression of myocardial contractile function [Citation5]. Reperfusion strategy is the latest treatment for MI [Citation6] and leads to reactivation of the electron transport chain, causing the generation of reactive oxygen species (ROS), which causing the opening of mitochondrial permeability transition pores, the intracellular Ca2+ overload, cell membrane lipid peroxidation and oxidative damage to DNA [Citation7]. Apoptosis, oxidative stress and inflammation are key procedures in MI [Citation5,Citation8]. Understanding the molecular participant of these procedures is essential for searching novel treatments of MI.
Circular RNAs (circRNAs) are a new class of non-coding RNAs, presenting a whole closed loop structure without poly-A tail [Citation9]. Due to their stability, evolutionary conservation, high abundance and specific expression, circRNA has been reported to be related to many physiological and pathological procedures [Citation10,Citation11]. Besides, many researches have studied that alterations in circRNA expression may lead to abnormal expression of gene products, contributing to cancer occurrence [Citation12]. CircRNAs have been verified as potential biomarkers in various malignancies, such as breast [Citation13], colon [Citation14] and gastric [Citation15] cancer. CircMAT2B, a novel circRNA, was proved to play positive role in regulating glucose metabolism under hypoxia [Citation16]. And it was up-regulated in hepatocellular carcinoma (HCC), took tumor-promoting role and could be a potential therapeutic goal in HCC. However, the role of circMAT2B in MI are unknown.
MicroRNAs (miRNAs) are a type of small non-coding RNAs, which can be sponged and regulated by circRNAs [Citation17]. Higher expression of miR-133 exists in the hearts of healthy adults contrasted with fetal hearts [Citation18]. It has been reported that miR-133 was dysregulated in infarcted tissue and distal myocardial samples in patients with MI [Citation19]. Moreover, miR-133 was proved to participate in the regulation of pathological processes of tumor through being regulated by circRNAs, such as ciRS-133 [Citation20] and circFUT10 [Citation21]. But, whether there is a regulatory mechanism between circMAT2B and miR-133 in MI is still unknown. Oxygen-glucose deprivation (OGD) is widely used to induce cellular model of ischemic heart damage in vitro [Citation22–24]. Besides, it is not only employed to elucidate the function of important cellular and molecular mechanisms behind cardiac ischemia but also to develop possible prevention strategies [Citation25]. Here, we used OGD to induce inflammatory damage in H9c2 cells and to explore the roles and regulatory mechanisms of circMAT2B in MI.
Materials and methods
Cell
Rat embryonic ventricular cardiomyocytes H9c2 cells were bought from ATCC (Manassas, VA, USA) and cultured in DMEM (GibcoBRL, Gaithersburg, MD, USA) with antibiotic mixture (100 U/ml penicillin and 100 μg/ml streptomycin) and 10% fetal bovine serum (FBS, HyClone, Logan, UT, USA) in a 37°C environment with 5% CO2.
Cell culture medium was substituted with serum-free and glucose-free DMEM. Then, cells were disclosed to hypoxic status (0.5% O2, 94% N2 and 5% CO2) in a 37°C environment at the specified time as the OGD group [Citation26]. H9c2 cells under normoxia were the control (CTRL).
qRT-PCR
RNA was gathered through TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) following the producer’s directions. Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland) was employed to carry out reverse transcription experiment. The qRT-PCR reactions were finished through SYBR Premix Ex TaqTM (Takara, Dalian, China) on an Applied Biosystems 7300 Real-time PCR system (Applied Biosystems, CA, USA) following the producer’s instructions. The inside standard control of circMAT2B and miR-133 was β-actin and U6, respectively. The sequences of primers were listed as follows: circMAT2B forward, 5ʹ-AATTGCAGATGCCTTCAACC-3ʹ, reverse, 5ʹ-TTCCCAGAAGCATCCACATT-3ʹ; miR-133 forward, 5ʹ-ACACTCCAGCTGGGAGCTGGTAAAAUGGAA-3ʹ, reverse, 5ʹ-TGGTGTCGTGGAGTCG-3ʹ; β-actin forward, 5ʹ-CCCATCTATGAGGGTTACGC-3ʹ, reverse, 5ʹ-TTTAATGTCACGCACGATTTC-3ʹ; U6 forward, 5ʹ-CTCGCTTCGGCAGCACA-3ʹ, reverse, 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ.
Transfection
Plasmid for circMAT2B overexpression (ex-circMAT2B), circMAT2B knockdown (si-circMAT2B) and the relative control were got from Genewiz (Suzhou, China). MiR-133 inhibitor and NC inhibitor were bought from OBIO Biotechnology Company (Shanghai, China). Transfections were done through Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). After 48 h, cells were harvested for latter analyses. The sequences used in this part were listed as follows: si-circMAT2B, 5ʹ-AAGACCTCCCCATGTTATAGT-3ʹ; si-NC, 5ʹ-TCCAGGAGAAATTACCGGAGA-3ʹ; miR-133 inhibitor, 5ʹ-CAGCUGGUUGAAGGGGACCAAA-3ʹ; NC inhibitor, 5ʹ-CGCGCACCCAAAUAAUUUUGGA-3ʹ.
CCK-8 experiment
CCK-8 (Dojindo, Japan) was employed to test cell activity following producer’s instruction. 1 × 104 cells were put in 96-well plates. Later, 10 μl CCK-8 solution was put into every well and cells were kept in a 37°C environment for 90 min. The 450 nm absorbance was examined and recorded through a microplate reader (Tecan, Männedorf, Switzerland).
Apoptosis experiment
The trypsinized and PBS-washed cells were re-suspended by 1 × binding buffer. Then, cells were stained by Annexin V-FITC/PI (BD Biosciences, San Diego, CA, USA). After incubating for 15 min, cells were analyzed through Flow cytometry analysis (FACScan, BD Biosciences).
ROS experiment
A ROS assay kit (Beyotime, Shanghai, China) was employed to detect ROS level. Cells were cleaned with phosphate-buffered saline (PBS) and cultured with 2,7-dichlorofluorescein diacetate (DCFH-DA, Nanjing Jiancheng, Nanjing, China) in a 37°C dark environment for 30 min. Then, cells were cleaned by PBS for 3 times. Fluorescent strengths were examined through a BD LSR flow cytometer (BD Biosciences, 488 nm excitation and 521 emission)
Enzyme-linked immunosorbent assay (ELISA)
Supernatant was gathered from 24-well plates. Concentrations of TNF-α, IL-6 and IL-1β were examined through ELISA kit (Elabscience Biotechnology Co., Ltd, Wuhan, China) following producer’s instruction and standardized to cell protein concentration.
Western blot
Protein was gathered via RIPA lysis buffer (Beyotime) and its concentration was measured through BCA Protein Assay Kit (Beyotime). Protein samples were separated through SDS-PAGE and then transferred onto PVDF membrane, which was closed by 5% nonfat dry milk in the room for 1 h. Next, membrane was cultured with primary antibodies all the night at 4°C. After that, membrane was washed and incubated with secondary antibody marked by horseradish peroxidase at room temperature for 1 h. The observation of positive blots was done through using enhanced chemiluminescence (ECL).
Statistics
Analyses were done through GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA, USA). Assays were done in triplicate. Data were shown as mean + SD. Differences were analyzed through t-test or one-way ANOVA. P value of significant result was set as P < 0.05.
Results
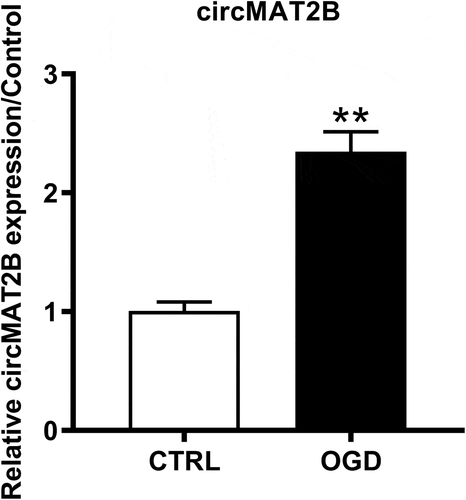
Increased expression of circMAT2B was induced by OGD in H9c2 cells
First, qRT-PCR was employed to detect circMAT2B expression levels in OGD-treated H9c2 cells. shows that circMAT2B expression is notably raised in OGD treatment group (P < 0.01).
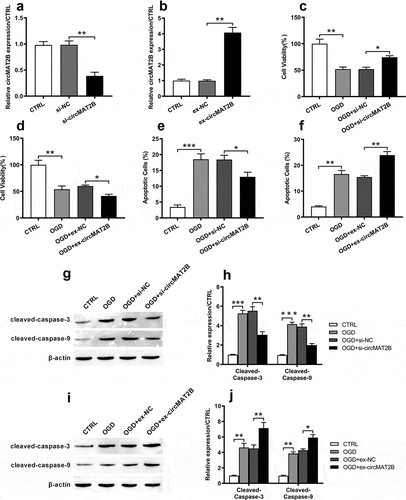
Knockdown of circMAT2B decreased OGD-induced apoptosis in H9c2 cells
Based on , we assumed that circMAT2B took important role in OGD-caused inflammatory injury. We next built up circMAT2B knockdown or overexpression cell model to explore the role of circMAT2B. As shown in ), circMAT2B expression was notably reduced by transfection with si-circMAT2B in H9c2 cells (P < 0.01). And circMAT2B overexpression cell model was also successfully established after transfection with ex-circMAT2B (P < 0.001, )). CCK-8 assay illustrated that OGD could significantly reduce cell viability (both P < 0.01, ,d)). CircMAT2B knockdown promoted cell activity (P < 0.05, )), while its overexpression notably suppressed cell activity (P < 0.05, )). Besides, flow cytometry revealed that OGD notably increased apoptosis (P < 0.001 and P < 0.01, ,f)). CircMAT2B knockdown notably reduced apoptosis (P < 0.05, )), while circMAT2B overexpression group was opposite (P < 0.01, )). Similar trends are occurred in –j) that the increasing levels of cleaved-caspase-3 and cleaved-caspase-9 caused by OGD were notably reduced by si-circMAT2B (both P < 0.01), while those were further raised by ex-circMAT2B (P < 0.01 and P < 0.05).
Figure 2. CircMAT2B knockdown decreased OGD-induced H9c2 cells’ apoptosis. CircMAT2B knockdown or overexpression were successfully established after transfection with si-circMAT2B (a) and ex-circMAT2B (b). (c, d) Cell activity was examined through CCK-8. (e, f) Apoptosis was examined via flow cytometry. (g–j) Levels of apoptosis-related factors were detected via western blot analysis. * P < 0.05, ** P < 0.01 and *** P < 0.001 compared to indicated group
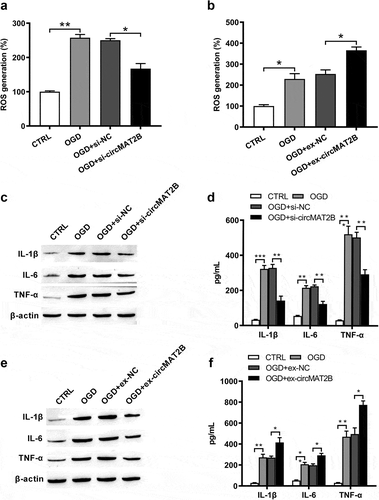
CircMAT2B knockdown decreased OGD-induced ROS generation and the expression of inflammatory factors
Then, OGD could notably raise ROS generation (P < 0.01 and P < 0.05, ,b)) and the expression of IL-1β (P < 0.001 and P < 0.01), IL-6 (both P < 0.01) and TNF-α (both P < 0.001, –f)) to enhance inflammation. ROS generation was notably reduced by circMAT2B knockdown (P < 0.05, )), while was further raised by circMAT2B overexpression (P < 0.05, )). Levels of IL-1β, IL-6 and TNF-α were significantly decreased by circMAT2B knockdown (all P < 0.01, ,d)), while were further increased in circMAT2B overexpression groups (P < 0.05, P < 0.05 and P < 0.01, ,f)).
Figure 3. CircMAT2B knockdown decreased OGD-induced ROS generation and release of inflammatory factors. H9c2 cells were transfected with si-circMAT2B and ex-circMAT2B. (a, b) ROS generation was examined via flow cytometry using DCFH-DA. Expression of IL-1β, IL-6 and TNF-α was examined via western blot (c, e) and ELISA (d, f). * P < 0.05, ** P < 0.01 and *** P < 0.001 contrasted with indicated set
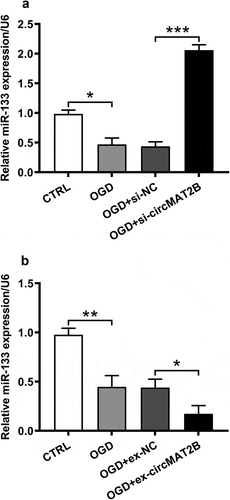
CircMAT2B negatively regulated miR-133
Previous study has proved that cardiac expression of miR-133 notably reduced in patients with myocardial infarction [Citation19]. And OGD could notably induce the reduced expression of miR-133 (P < 0.05, )). Furthermore, we observed that miR-133 expression was apparently raised by circMAT2B knockdown (P < 0.001, )), while was obviously decreased by circMAT2B overexpression (P < 0.05, )).
CircMAT2B knockdown reduced inflammatory injury caused by OGD in H9c2 cells via up-regulating miR-133
Based on , we assumed that the inhibition of miR-133 might eliminate and reverse the effects of circMAT2B knockdown. To further explore the function of miR-133 in the effects of circMAT2B, miR-133 inhibitor was transfected into H9c2 cells. The expression of miR-133 was dramatically decreased after transfection with miR-133 inhibitor (P < 0.01, )). Inhibition of miR-133 reduced the increasing of cell viability caused by si-circMAT2B (P < 0.05, )). Besides, the reducing apoptosis induced by si-circMAT2B was eliminated and slightly increased after transfection with miR-133 inhibitor (P < 0.05, )). The same tendency occurred in the detection of cleaved-caspase-3 and cleaved-caspase-9 (,e)). Moreover, miR-133 inhibitor blocked the si-circMAT2B-caused inhibitory effects on ROS generation (P < 0.05, )) and the expression of IL-1β, IL-6 and TNF-α (all P < 0.05, ,h)).
Figure 5. CircMAT2B knockdown decreased OGD-induced H9c2 cells inflammatory injury via up-regulating miR-133. (a) Level of miR-133 was examined via qRT-PCR after transfection with miR-133 inhibitor. (b) Cell viability was examined through CCK-8. (c) Apoptosis was examined through flow cytometry. (d,e) Levels of apoptosis-related factors were examined through western blot analysis. (f) ROS generation was examined via flow cytometry using DCFH-DA. Expression of IL-1β, IL-6 and TNF-α was examined via western blot (g) and ELISA (h). * P < 0.05, ** P < 0.01 and *** P < 0.001 contrasted with indicated set
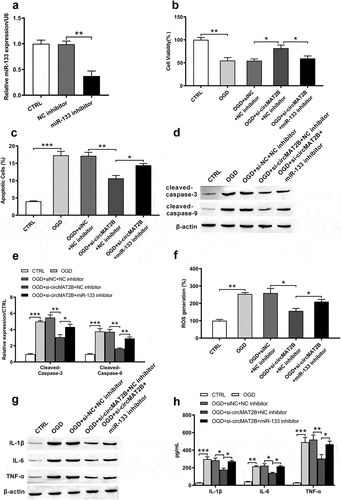
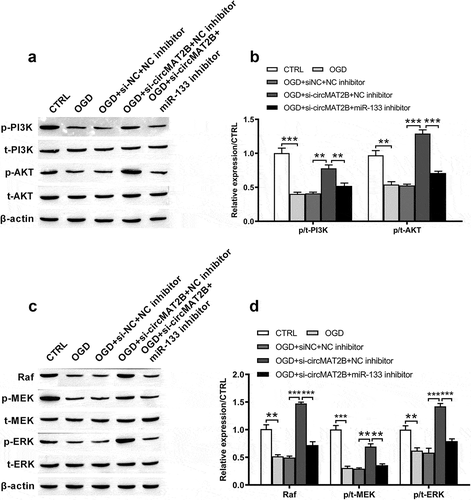
CircMAT2B knockdown attenuated OGD-induced the inhibition of PI3K/AKT and Raf/MEK/ERK pathways through up-regulating miR-133
PI3K/AKT signal pathway is considerable in defending against myocardial ischemia reperfusion damage [Citation27]. Raf/MEK/ERK pathway takes key functions in cell development and inflammatory response [Citation28,Citation29]. We tested the functional mechanism of circMAT2B in these pathways. From –d), levels of p/t-PI3K (P < 0.001), p/t-AKT (P < 0. 01), Raf (P < 0.01), p/t-MEK (P < 0.001) and p/t-ERK (P < 0. 01) were notably decreased by OGD. They were all notably increased by circ-MAT2B knockdown (P < 0.01, P < 0.001, P < 0.001, P < 0.01 and P < 0.001). However, these promoting effects were reversed by the adding of miR-133 inhibitor (P < 0.01, P < 0.001, P < 0.001, P < 0.01 and P < 0.001, –d)).
Figure 6. CircMAT2B knockdown attenuated OGD-induced the inhibition of PI3K/AKT and Raf/MEK/ERK pathways via positively regulating miR-133 in H9c2 cells, which were co-transfected with si-circMAT2B and miR-133 inhibitor. (a-b) Levels of p/t-PI3K and p/t-AKT were examined through western blot analysis. (c-d) Levels of Raf, p/t-MEK and p/t-ERK were examined through western blot analysis. ** P < 0.01 and *** P < 0.001 contrasted with indicated set
Discussion
MI is proved to be accompanied by a notably higher inflammatory activity related to induction of IL-6 [Citation30]. It is characterized by ischemic damage and cardiomyocyte cell death, causing heart failure. However, reperfusion strategy could cause cardiomyocytes death, and there is no effective method to inhibit myocardial reperfusion damage [Citation5]. CircMAT2B is a novel circRNA and is proved to be positive in regulating glucose metabolism under hypoxia. The present research firstly uncovered the role of circMAT2B in OGD-caused H9c2 cell inflammatory injury. CircMAT2B was highly expressed by OGD treatment. Besides, circMAT2B knockdown could decrease OGD-induced apoptosis, ROS generation and expression of IL-1β, IL-6, TNF-α and the inhibition of PI3K/AKT and Raf/MEK/ERK pathways via positively regulating miR-133. These findings suggested the anti-inflammatory role of circMAT2B knockdown in OGD-induced H9c2 cells inflammatory damage.
As a special class of non-coding RNA, circRNAs’ functions in carcinogenesis and cancer progression have now attracted widespread attention. They are different from the linear RNAs for lacking of 5ʹ or 3ʹ tails [Citation31]. Recent researches have reported that the dysregulation of circRNAs elevates the development of many cancers [Citation32,Citation33]. Besides, for OGD inducement, Lin et al. investigated the circRNA profile of mouse hippocampal OGD HT22 cells model and identified a few cirRNAs that might take part in metabolism, growth and immunoreaction [Citation34]. Jin et al. observed that silencing circ_0010729 could protect cardiomyocytes from OGD-caused damage via up-regulating miR-145-5p [Citation35]. CircMAT2B, a novel circRNA, has been proved to play tumor-promoting roles in HCC [Citation16]. Its overexpression blocks apoptosis and elevates HCC cell growth, invasion and migration under hypoxia. Moreover, it was verified as a positive regulator in glucose metabolism under hypoxia. For the first time, we observed that circMAT2B was highly expressed after OGD treatment, indicating the monitoring role of circMAT2B in OGD-caused inflammation. Similar to the function of circ_0010729 in OGD-caused cardiomyocytes damage, knockdown of circMAT2B played anti-inflammatory role in OGD-induced H9c2 cell inflammatory injury. CircMAT2B knockdown decreased OGD-induced apoptosis, ROS generation, the emancipation of IL-1β, IL-6, TNF-α and the inhibition of PI3K/AKT and Raf/MEK/ERK pathways via positively regulating miR-133. This discovery offered a potential goal for MI treatment.
It has been reported that miRNAs take central roles in regulating key protein-coding genes associated with cardiovascular disease [Citation36–38]. MiR-133 was recently shown to be involved in the processes of angiogenesis and regeneration of cardiomyocytes [Citation36]. Cardiac expression of miR-133 was notably decreased in patients with MI [Citation19]. However, circulating miR-133 expression was proved to be increased in plasma and whole blood of acute myocardial infarction (AMI) patients contrasted with that in control subjects and can be a novel biomarker for AMI detection [Citation39–41]. These reports revealed its tissue-specificity. Besides, miR-133 exerted defense role against oxidative stress-caused cardiomyocyte apoptosis through negatively regulating caspase-3 and caspase-9 [Citation42], and its up-regulation improved the fibrosis and inflammation in infarcted hearts [Citation18]. Consistently, we observed that inhibition of miR-133 prevented the inhibitory effects of circMAT2B knockdown on OGD-induced apoptosis, cleaved-caspase-3 and cleaved-caspase-9, ROS generation and inflammatory factors, suggesting the potential anti-inflammatory roles of miR-133. Furthermore, circRNAs can sponge miRNA to regulate transcription and post-transcription. For example, ciRS-133 elevates the differentiation of preadipocytes into brown-like cells through actuating PRDM16 and inhibiting miR-133 [Citation20]. CircFUT10 modulates myoblast differentiation and cell survival through directly binding to miR-133 and down-regulating miR-133a expression [Citation21]. Innovatively, we firstly found the regulatory mechanism between circMAT2B and miR-133 that circMAT2B took anti-inflammatory roles in OGD-induced H9c2 cells inflammatory injury via up-regulating miR-133. This mechanism provides theoretical basis for circMAT2B in treating MI. Further work is needed to investigate the sponge relationship between circMAT2B and miR-133 to enrich this regulatory mechanism.
As we all know, PI3K/AKT pathway is a key regulatory pathway for protein synthesis in vivo and is closely associated with the oxidation and reduction of intracellular mitochondria [Citation43]. It was proved to be inhibited in myocardial cells of rats with MI, causing the apparent increase of myocardial apoptosis [Citation44]. The Raf/MEK/ERK pathway is one of the most characteristic pathways in the MAPK cascades and it can regulate responses in cardiomyocytes [Citation45,Citation46]. Recent study has found that circRNAs take important roles in regulating PI3K/AKT and Raf/MEK/ERK pathways. For example, hsa-circ-0072309 plays anti-tumor role in renal carcinoma cells through blocking PI3K/AKT pathway [Citation47]. circRNA_0006528 promotes the development of breast cancer through activating MAPK/ERK pathway [Citation48]. However, whether circMAT2B is involved in regulating these pathways is unknown. Our study firstly demonstrated the regulatory mechanism among circMAT2B, miR-133 and PI3K/AKT and Raf/MEK/ERK pathways. Knockdown of circMAT2B could promote the activation of PI3K/AKT and Raf/MEK/ERK pathways through up-regulating miR-133. These findings further suggested the functional mechanism of circMAT2B.
In conclusion, our research initially explored the roles of circMAT2B in OGD-induced H9c2 cells inflammatory damage. The findings indicated that circMAT2B was highly expressed by OGD treatment and knockdown of circMAT2B reduced OGD-induced apoptosis, ROS production, release of inflammatory factors and the inhibition of PI3K/AKT and Raf/MEK/ERK pathways. Mechanistically, knockdown of circMAT2B played anti-inflammatory role through up-regulating miR-133, which might offer a new therapeutic goal for monitoring and treating MI.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Heusch G. Myocardial ischemia: lack of coronary blood flow or myocardial oxygen supply/demand imbalance? Circ Res. 2016;119:194–196.
- Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21:365–371.
- Shindo Y, Kanak MA. Regulation of Inflammatory Response in Islet Transplantation. OBM Transplant. 2018;2:013.
- Lu L, Liu M, Sun R, et al. Myocardial infarction: symptoms and treatments. Cell Biochem Biophys. 2015;72:865–867.
- Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100.
- Bell RM, Botker HE, Carr RD, et al. 9th hatter biannual meeting: position document on ischaemia/reperfusion injury, conditioning and the ten commandments of cardioprotection. Basic Res Cardiol. 2016;111:41.
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135.
- Penberthy JK, Chhabra D, Avitabile N, et al. Mindfulness based therapies for autoimmune diseases and related symptoms. OBM Integ Complement Med. 2018;3:039.
- Su Y, Xu C, Liu Y, et al. Circular RNA hsa_circ_0001649 inhibits hepatocellular carcinoma progression via multiple miRNAs sponge. Aging (Albany NY). 2019;11:3362–3375.
- Li Y, Zheng F, Xiao X, et al. CircHIPK3 sponges miR-558 to suppress heparanase expression in bladder cancer cells. EMBO Rep. 2017;18:1646–1659.
- Han D, Li J, Wang H, et al. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66:1151–1164.
- Chen L, Zhang S, Wu J, et al. circRNA_100290 plays a role in oral cancer by functioning as a sponge of the miR-29 family. Oncogene. 2017;36:4551–4561.
- He R, Liu P, Xie X, et al. circGFRA1 and GFRA1 act as ceRNAs in triple negative breast cancer by regulating miR-34a. J Exp Clin Cancer Res. 2017;36:145.
- Hsiao KY, Lin YC, Gupta SK, et al. Noncoding effects of circular RNA CCDC66 promote colon cancer growth and metastasis. Cancer Res. 2017;77:2339–2350.
- Zhang J, Liu H, Hou L, et al. Circular RNA_LARP4 inhibits cell proliferation and invasion of gastric cancer by sponging miR-424-5p and regulating LATS1 expression. Mol Cancer. 2017;16:151.
- Li Q, Pan X, Zhu D, et al. Circular RNA MAT2B promotes glycolysis and malignancy of hepatocellular carcinoma through the miR-338-3p/PKM2 axis under hypoxic stress. Hepatology. 2019;70:1298–1316.
- Wu Y, Xie Z, Chen J, et al. Circular RNA circTADA2A promotes osteosarcoma progression and metastasis by sponging miR-203a-3p and regulating CREB3 expression. Mol Cancer. 2019;18:73.
- Chen Y, Zhao Y, Chen W, et al. MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res Ther. 2017;8:268.
- Bostjancic E, Zidar N, Stajer D, et al. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology. 2010;115:163–169.
- Zhang H, Zhu L, Bai M, et al. Exosomal circRNA derived from gastric tumor promotes white adipose browning by targeting the miR-133/PRDM16 pathway. Int J Cancer. 2019;144:2501–2515.
- Li H, Yang J, Wei X, et al. CircFUT10 reduces proliferation and facilitates differentiation of myoblasts by sponging miR-133a. J Cell Physiol. 2018;233:4643–4651.
- Lv B, Hua T, Li F, et al. Hypoxia-inducible factor 1 alpha protects mesenchymal stem cells against oxygen-glucose deprivation-induced injury via autophagy induction and PI3K/AKT/mTOR signaling pathway. Am J Transl Res. 2017;9:2492–2499.
- Liu J, Yang S, Zhang X, et al. Isoflurane reduces oxygen-glucose deprivation-induced oxidative, inflammatory, and apoptotic responses in H9c2 cardiomyocytes. Am J Transl Res. 2016;8:2597.
- Chiang MH, Liang CJ, Liu CW, et al. Aliskiren improves ischemia- and oxygen glucose deprivation-induced cardiac injury through activation of autophagy and amp-activated protein kinase. Front Pharmacol. 2017;8:819.
- Li Y, Du L, Cheng S, et al. WNT3A aggravates oxygen-glucose deprivation-induced injury in h9c2 cardiomyocytes via inhibition of SIRT3. Am J Clin Cardiol. 2019;1:1006.
- Karuppagounder SS, Basso M, Sleiman SF, et al. In vitro ischemia suppresses hypoxic induction of hypoxia-inducible factor-1alpha by inhibition of synthesis and not enhanced degradation. J Neurosci Res. 2013;91:1066–1075.
- Lu C, Wang X, Ha T, et al. Attenuation of cardiac dysfunction and remodeling of myocardial infarction by microRNA-130a are mediated by suppression of PTEN and activation of PI3K dependent signaling. J Mol Cell Cardiol. 2015;89:87–97.
- Zhang J, Lan SJ, Liu QR, et al. Neuroglobin, a novel intracellular hexa-coordinated globin, functions as a tumor suppressor in hepatocellular carcinoma via Raf/MAPK/Erk. Mol Pharmacol. 2013;83:1109–1119.
- Hsieh YH, Deng JS, Chang YS, et al. Ginsenoside Rh2 ameliorates lipopolysaccharide-induced acute lung injury by regulating the TLR4/PI3K/Akt/mTOR, Raf-1/MEK/ERK, and Keap1/Nrf2/HO-1 signaling pathways in mice. Nutrients. 2018;10:1208.
- Hashmi S, Al-Salam S. Acute myocardial infarction and myocardial ischemia-reperfusion injury: a comparison. Int J Clin Exp Pathol. 2015;8:8786–8796.
- Toptan T, Abere B. Circular DNA tumor viruses make circular RNAs. Proc Natl Acad Sci U S A. 2018;115:E8737–e45.
- Ng WL, Mohd Mohidin TB, Shukla K. Functional role of circular RNAs in cancer development and progression. RNA Biol. 2018;15:995–1005.
- Si-Tu J, Cai Y, Feng T, et al. Upregulated circular RNA circ-102004 that promotes cell proliferation in prostate cancer. Int J Biol Macromol. 2019;122:1235–1243.
- Lin SP, Ye S, Long Y, et al. Circular RNA expression alterations are involved in OGD/R-induced neuron injury. Biochem Biophys Res Commun. 2016;471:52–56.
- Jin Q, Chen Y. Silencing circular RNA circ_0010729 protects human cardiomyocytes from oxygen-glucose deprivation-induced injury by up-regulating microRNA-145-5p. Mol Cell Biochem. 2019;462:185–194.
- Navickas R, Gal D, Laucevicius A, et al. Identifying circulating microRNAs as biomarkers of cardiovascular disease: a systematic review. Cardiovasc Res. 2016;111:322–337.
- Schober A, Weber C. Mechanisms of MicroRNAs in atherosclerosis. Annu Rev Pathol. 2016;11:583–616.
- Gómez-Dos-Santos V, Hevia-Palacios V, Ramos-Muñoz E, et al. MicroRNAs as potential markers for advantageous perfusion in a preclinical donation after cardiac death animal model of oxygenated hypothermic machine perfusion (HOPE). OBM Transplant. 2018;2:012.
- Wang R, Li N, Zhang Y, et al. Circulating microRNAs are promising novel biomarkers of acute myocardial infarction. Intern Med. 2011;50:1789–1795.
- Peng L, Chun-guang Q, Bei-fang L, et al. Clinical impact of circulating miR-133, miR-1291 and miR-663b in plasma of patients with acute myocardial infarction. Diagn Pathol. 2014;9:89.
- Li C, Pei F, Zhu X, et al. Circulating microRNAs as novel and sensitive biomarkers of acute myocardial Infarction. Clin Biochem. 2012;45:727–732.
- Xu C, Hu Y, Hou L, et al. beta-Blocker carvedilol protects cardiomyocytes against oxidative stress-induced apoptosis by up-regulating miR-133 expression. J Mol Cell Cardiol. 2014;75:111–121.
- Chi Y, Ma Q, Ding X, et al. Research on protective mechanism of ibuprofen in myocardial ischemia-reperfusion injury in rats through the PI3K/Akt/mTOR signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:4465–4473.
- Yu P, Zhang J, Yu S, et al. Protective effect of sevoflurane postconditioning against cardiac ischemia/reperfusion injury via ameliorating mitochondrial impairment, oxidative stress and rescuing autophagic clearance. PLoS One. 2015;10:e0134666.
- Widmann C, Gibson S, Jarpe MB, et al. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180.
- Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002;91:776–781.
- Chen T, Shao S, Li W, et al. The circular RNA hsa-circ-0072309 plays anti-tumour roles by sponging miR-100 through the deactivation of PI3K/AKT and mTOR pathways in the renal carcinoma cell lines. Artif Cells Nanomed Biotechnol. 2019;47:3638–3648.
- Gao D, Qi X, Zhang X, et al. hsa_circRNA_0006528 as a competing endogenous RNA promotes human breast cancer progression by sponging miR-7-5p and activating the MAPK/ERK signaling pathway. Mol Carcinog. 2019;58:554–564.