ABSTRACT
The present study aimed to assess the role of miR-1275 in cardiac ischemia reperfusion injury. H9 human embryonic stem cell (hESC)-derived cardiomyocytes stimulated by oxygen-glucose deprivation/reoxygenation (OGD/R) were used to simulate myocardial injury in vitro. miR-1275 expression levels in cells were measured by RT-qPCR. The release of lactate dehydrogenase (LDH) and creatine kinase (CK) was examined through LDH and CK ELISA kits. Cell apoptosis was detected through flow cytometry. A Fura-2 Calcium Flux Assay Kit and a Fluo-4 assay kit were used to determine the Ca2+ concentration. Expression levels of proteins were tested by Western blotting. The binding effect of miR-1275 and neuromedin U type 1 receptor (NMUR1) was detected by dual-luciferase activity assay. The results showed that miR-1275 was upregulated in OGD/R-stimulated cardiomyocytes. Inhibition of miR-1275 suppressed the increased activity of LDH and CK, cell apoptosis, reactive oxygen species (ROS) production, intracellular Ca2+ concentration and sarcoplasmic reticulum (SR) Ca2+ leak induced by OGD/R treatment in cardiomyocytes. miR-1275 directly targets 3ʹUTR of NMUR1 and negatively regulates NMUR1 expression. Silence of NMUR1 abolished the protecting effect of the miR-1275 antagomir on myocardial OGD/R injury. Our study indicated that the miR-1275 antagomir protects cardiomyocytes from OGD/R injury through the promotion of NMUR1.
KEYWORDS:
1. Introduction
Cardiac ischemia reperfusion injury (CIRI) is an unavoidable event during cardiac surgery and is associated with postoperative morbidity and mortality [Citation1]. Among pediatric disorders, congenital heart disease, cardiomyopathy, congestive heart failure, infant and neonatal myocardial infarction and other cardiovascular diseases can lead to CIRI directly or indirectly [Citation2–4]. For instance, myocardial injury caused by cardiopulmonary bypass and cardioplegic arrest during cardiac surgery [Citation5]. For decades, clinicians are committed to finding effective and clinically practical strategies to protect the heart from ischemia reperfusion injury. This highlighted the need for understanding the mechanisms underlying this phenomenon.
MicroRNAs (miRNAs) are a class of small and noncoding RNAs (~22 nucleotides in length) that participated in the development of many diseases, such as cancer, inflammation, and cardiovascular disease [Citation6,Citation7]. Accumulating evidence suggests that miRNAs serve as post-transcriptional regulators of genes, repressing gene expression through their binding at the complementary 3´ untranslated region (3´ UTR) [Citation8]. The involvement of miRNAs in CIRI and conditioning has been intensively studied in myocardial infarction [Citation9]. A number of miRNAs (miR‐31, miR‐26a, miR‐17-3p, miR‐199a, miR‐1214) have been reported to be regulated by ischemia-reperfusion injury [Citation10–13]. miR-1275 has been reported to participate in the development of hepatocellular carcinoma, nasopharyngeal tumors, and lung cancer [Citation14–16]. In addition, it has been reported that miR-1275 was downregulated in obese subjects and inhibited adipogenesis [Citation17,Citation18]. It was also found that serum miR-1275 was upregulated in women pregnant with fetuses with congenital heart defects [Citation19], and increased in congenital heart defect children (children <1 year of age) [Citation20]. What is more, recently reports found that miR-1275 was upregulated in acute ischemic stroke patients, and increased in heart tissues after cardiopulmonary bypass and cardioplegic, even though protected through ischemic postconditioning [Citation5,Citation21]. However, its specific role in the progression of pediatric CIRI remains unknown.
Previous reports indicated that ischemia-reperfusion injury is associate with unregulated calcium overload [Citation22]. Defective Ca2+ handling serves as an essential pathophysiological mechanism in ischemia-reperfusion injury [Citation23]. Hence, the effect of miR-1275 on cell injury, apoptosis, oxidative stress, and calcium overload in oxygen-glucose deprivation/reoxygenation (OGD/R) stimulated cardiomyocytes were explored in this study.
2. Materials and methods
2.1. Cell lines and treatment
The H9 human embryonic stem cell (hESC) line and rat cardiomyocytes H9C2 cell lines were purchased from Stem Cell Bank, Chinese Academy of Sciences (Shanghai, China). H9C2 cells were cultured in DMEM culture medium with 10% fetal bovine serum (FBS, Gibco). hESCs were cocultured with irradiated mouse embryonic fibroblast feeder cells in KnockoutTM DMEM culture medium (Gibco) and 20% KnockOutTM Serum Replacement (Gibco) as a previous study reported [Citation24]. In addition, L-glutamine (2 mM), nonessential amino acids (0.1 mM), β-mercaptoethanol (0.1 mM), and recombinant human FGF-basic (15 ng/mL) were added to the culture. To induce cardiomyocyte differentiation, hESC colonies were dispersed into small clumps and transferred to plastic petri dishes, cultured with KnockoutTM DMEM culture medium containing 20% defined fetal bovine serum (Gibco), 2 mM glutamine, 0.1 mM nonessential amino acids, and 0.1 mM β-mercaptoethanol. While they were cultured in the suspension for 7 days, the cells were aggregated to form embryoid bodies (EBs) [Citation25]. Then, the EBs were attached to 0.1% gelatine-coated plates and allowed to differentiate for an additional 14 days. Furthermore, cardiomyocyte-like cells were isolated from the EBs and cultured in 0.1% gelatine-coated plates for 24 hours.
The in vitro model of myocardial OGD/R injury was performed as previously described [Citation26]. HESC-derived cardiomyocytes and H9C2 cells were stimulated by oxygen and glucose deprivation, followed by reperfusion [Citation26]. Cardiomyocytes were cultured in serum and glucose-deficient DMEM and subjected to hypoxic conditions (1% O2 and 5% CO2 at 37°C for 12 h). Afterward, cardiomyocytes were re-oxygenated through culturing in complete high-glucose DMEM medium containing 10% FBS for 6 h. Control group cells were cultured in normal high-glucose DMEM medium in 5% CO2 at 37°C.
2.2. Assessment of cardiomyocyte injury
The levels of LDH (lactate dehydrogenase, an indicator of cell injury) and CK (creatine kinase, a marker for myocardial infarction) in the culture medium of OGD/R injury-stimulated cells and control cells were examined through LDH and CK ELISA kits, according to the manufacturer’s protocol (Institute of Jiancheng Biotechnology, China) [Citation27].
2.3. RNA extraction and reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Cells were harvested through tryptic digestion for 10 min at 37°C. Then, total RNAs from cells were isolated by Trizol reagent according to the manufacturer’s instructions (Invitrogen Carlsbad, CA, USA). The content of miR-1275 and NMUR1 was examined by RT-qPCR, as previously reported [Citation28]. The primers of miR-1275 and NMUR1 were synthesized by GenePharma (Shanghai, China).
2.4. Construction and transduction of the miR-1275 antagomir, miR-1275 mimics, and si-NMUR1
The mimic and antagomir of miR-1275 were synthesized and purchased from GenePharma. The siRNA of NMUR1 (si-NMUR1) was constructed and purchased from Generay Biotech (Shanghai, China). LipofectamineTM 3000 was used to transfect miR-1275 mimics, the miR-1275 antagomir, and si-NMUR1 into cells, according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA).
2.5. Luciferase assay
Wild-type and mutant 3′UTR fragments of the NMUR1 gene were cloned into the pGL3 luciferase reporter vector (Promega, Madison, Wisconsin, WI, USA), named LUC-WT-NMUR1 (Wild-type NMUR1 luciferase reporter vector) and LUC-MUT-NMUR1 (Mutant-type NMUR1 luciferase reporter vector). The miR-1275 mimics and LUC-WT-NMUR1 or LUC-MUT-NMUR1 were both transfected into cells for 48 h. Luciferase activities were assessed using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s protocol.
2.6. Cell apoptosis test
Cell apoptosis was determined using an Annexin V-FITC/PI double staining kit (Beyotime, Jiangsu, China). Cells were harvested and rinsed with PBS. Then, binding buffer (500 μL) and Annexin V (10 μL) were added, and they were incubated in the dark for 20 min. Before analysis by flow cytometry, 5 μL of PI regents was added. Each sample was assayed in triplicate.
2.7. Reactive oxygen species (ROS) level
ROS level in cardiomyocytes was examined by the Cellular ROS/Superoxide detection assay kit following the manufacturer’s protocol (Abcam, UK).
2.8. Ca2+ concentration measurements
The concentration of intracellular Ca2+ was measured by the Fura-2 Calcium Flux Assay Kit (ab176766, Abcam, Cambridge, UK) according to the manufacturer’s protocol. Fura-2 reagents were added to the cells, and they were incubated for 1 h at 37°C and for another 20 min at room temperature. They were then analyzed by a microplate reader at 340 nm and 380 nm. The Fluo-4 Assay Kit (ab228555, Abcam, Cambridge, UK) was adopted to measure the SR Ca2+ leak, performed as previously described [Citation29].
2.9. Western blotting
Cells were harvested and lysed with RIPA lysis buffer on ice. Proteins were separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane. Then, protein bands were blocked with 5% skim milk for 1 h at room temperature and incubated with primary antibodies overnight at 4°C and the corresponding horseradish peroxidase-linked secondary antibody for 2 h at room temperature. In the end, the signals of the protein bands were detected using a SuperSignal West Pico Chemiluminescent Substrate kit according to the manufacturer’s instructions (Pierce Biotechnology, Inc., Rockford, IL, USA).
2.10. Data analysis
Data are presented as the mean ± SEM. Statistical significance was analyzed by one-way ANOVA and the student t-test using GraphPad Prism 5.0 software. P < 0.05 was considered significant.
3. Results
3.1. miR-1275 was upregulated in hESC-derived cardiomyocytes after treated by OGD/R
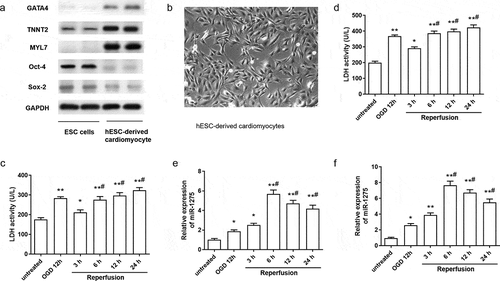
To confirm that human embryonic stem cells (hESCs) were differentiated into cardiomyocytes, specific cardiomyocyte markers and embryonic stem cells (ESC) specific markers were examined through western blotting. As shown in ), the specific cardiomyocyte markers GATA4, TNNT2, and MYL7 were highly expressed in hESC-derived cardiomyocytes compared with undifferentiated hESCs. Meanwhile, hESC-specific markers Oct-4 and Sox-2 were markedly decreased in hESC-derived cardiomyocytes compared with undifferentiated hESCs. The representative image of hESC-derived cardiomyocytes is displayed in ). For exploring the role of miR-1275 in CIRI, an in vitro model of myocardial OGD/R injury was established. The results suggested that the viability of cardiomyocytes was significantly decreased with prolonged reperfusion time ()). In addition, we found the expression of miR-1275 was increased in hESC-derived cardiomyocytes and rat H9C2 cardiomyocytes after treated by OGD for 12 h, and further enhanced after prolonged reperfusion time ()). And the expression of miR-1275 reached a peak at reperfusion 6 h ()). Hence, cardiomyocytes treated by OGD for 12 h and reperfusion for 6 h were adopted in the follow-up experiment.
Figure 1. miR-1275 was upregulated in hESC-derived cardiomyocytes and H9C2 cardiomyocytes after treated by OGD/R. (a) The expression of specific cardiomyocyte markers (GATA4, TNNT2, and MYL7) and hESC-specific markers (Oct-4 and Sox-2) in hESC-derived cardiomyocytes was examined by western blotting. (b) Representative image of hESC-derived cardiomyocytes. (c and d) LDH activity of hESC-derived cardiomyocytes and H9C2 cardiomyocytes was measured after treated by oxygen-glucose deprivation (OGD) followed by reperfusion for 3 h, 6 h, 12 h, 24 h. (e and f) The expression of miR-1275 in cardiomyocytes was measured through RT-qPCR after stimulation by OGD/R treatment. Values are expressed as mean ± SEM, n = 3, *P < 0.05, **P < 0.01, compared with the untreated group. #P < 0.05, compared with the OGD + reperfusion for 3 h treatment group
3.2. Knockdown of miR-1275 inhibited cell injury, cell apoptosis, oxidative stress, and calcium overloading in hESC-derived cardiomyocytes with OGD/R
To explore the effect of miR-1275 downregulation on OGD/R-induced myocardial injury, miR-1275 antagomirs were transfected into hESC-derived cardiomyocyte. The results suggested that miR-1275 antagomirs effectively inhibited the expression of miR-1275 in cardiomyocytes ()). While miR-1275 antagomir 1 (anta-miR1) has a higher inhibition efficiency in cardiomyocytes than miR-1275 antagomir 2 (anta-miR1). Therefore, miR-1275 antagomir 1 was adopted in the following experiments. Next, we found inhibition of miR-1275 markedly inhibited the increase of miR-1275 in cardiomyocytes that induced by OGD/R treatment ()). Meanwhile, inhibition of miR-1275 significantly suppressed the increase of LDH and CK in cardiomyocytes that induced by OGD/R treatment ()). In addition, the results of Annexin V/PI double stain assay showed that knockdown of miR-1275 prominently suppressed cell apoptosis induced by OGD/R treatment in cardiomyocytes ()). Besides, apoptosis-related proteins were measured. The results have shown that cleaved caspase 3, cytochrome C and Bax were increased in OGD/R treated cardiomyocytes, while miR-1275 inhibition suppressed these increases ()). The expression of antiapoptotic factor Bcl-2 was decreased in OGD/R stimulated cardiomyocytes and increased after miR-1275 upregulation ()). Next, the results displayed that ROS level was increased in OGD/R stimulated cardiomyocytes ()). Inhibition of miR-1275 suppressed the increase of ROS in cardiomyocytes that induced by OGD/R stimulation ()). Furthermore, we found that OGD/R treatment markedly increased the intracellular Ca2+ concentration and SR Ca2+ leak in cardiomyocytes, while inhibition of miR-1275 significantly suppressed the intracellular Ca2+ concentration and SR Ca2+ leak in cardiomyocytes ()). These results suggested that inhibition of miR-1275 reversed cell injury, apoptosis, oxidative stress and intracellular Ca2+ overload in OGD/R -stimulated cardiomyocytes.
Figure 2. Knockdown of miR-1275 inhibited cell apoptosis and cell injury in cardiomyocytes with OGD/R treatment. (a) Transfection efficiency of miR-1275 antagomirs in hESC-derived cardiomyocytes was examined. miR-1275 antagomirs (40 nM, anta-miR) or negative control antagomir (40 nM, anta-NC) were transfected into hESC-derived cardiomyocytes and cultured for 48 h. *P < 0.05, **P < 0.01, compared with negative control group. (b) The expression of miR-1275 in OGD/R-stimulated cardiomyocytes was measured after transfected with miR-1275 antagomir1 (anta-miR). (c, d) LDH and CK activity in OGD/R-stimulated cardiomyocytes were analyzed by ELISA Kits. (e, f) Cell apoptosis was detected through the Annexin V-FITC/PI double staining kit. (g) The expression of apoptosis-related proteins was detected through Western blotting. (h-j) ROS levels (h), Intracellular Ca2+ concentration (i) and SR Ca2+ leak (j) in cardiomyocytes were tested. Values are expressed as mean ± SEM, n = 3, *P < 0.05, **P < 0.01, compared with untreated group; #P < 0.05, compared with OGD/R + anta-NC treated group
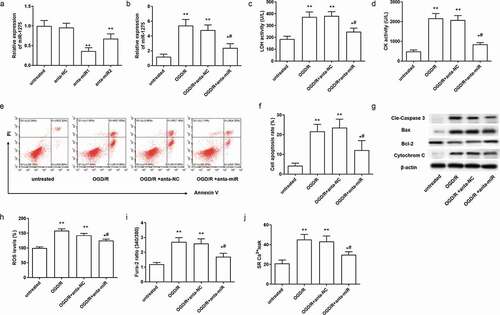
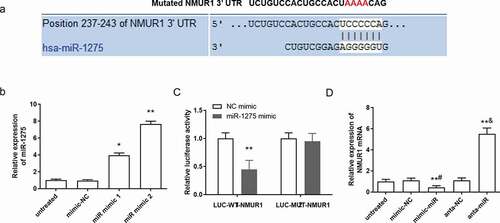
Figure 3. NMUR1 is a predicted target of miR-1275 and it is negatively regulated by miR-1275. (a) The miR-1275 seed sequences and the predicted binding sites of NMUR1 3ʹ-UTR mutation sites are highlighted in red. (b) Transfection efficiency of miR-1275 mimics in hESC-derived cardiomyocytes was examined. Negative control mimics (30 nM, mimic-NC) and miR-1275 mimics (30 nM, miR mimic) were transfected into hESC-derived cardiomyocytes and incubated for 48 h. (c) Luciferase activity of NMUR1 was tested. **P < 0.01, compared with NC mimic transfection group. (d) The expression of NMUR1 mRNA was examined. Values are expressed as mean ± SEM, n = 3, **P < 0.01, compared with untreated group. #P < 0.05, compared with mimic-NC transfected group; &P < 0.05, compared with anta-NC transfected group
3.3. Inhibition of miR-1275 promotes the expression of NMUR1
According to the prediction by TargetScan, there was a potential binding site in the 3ʹUTR region of NMUR1 with the miR-1275 sequence ()). The targeting effect of miR-1275 on the 3ʹUTR region of NMUR1 in cardiomyocytes was detected. MiR-1275 mimic 1 and mimic 2 were transfected into cardiomyocytes, the results suggested that miR-1275 mimic 2 has a higher promoting efficiency in cardiomyocytes than miR-1275 mimic 1 ()). Therefore, miR-1275 mimic 2 was adopted in the following experiments. The luciferase activity assay results showed that miR-1275 mimics markedly decreased the fluorescence signal intensity of NMUR1 in the LUC-WT-NMUR1 (Wild-type NMUR1 luciferase reporter vector) transfection group, whereas there was a non-significant effect on NMUR1 in the LUC-MUT-NMUR1 (Mutant-type NMUR1 luciferase reporter vector) transfection group ()). Meanwhile, miR-1275 mimics significantly decreased the expression of the NMUR1, whereas miR-1275 antagomir transfection increased the expression of the NMUR1 in cardiomyocytes ()). These results suggested that NMUR1 was a direct target of miR-1275 in cardiomyocytes and miR-1275 negatively regulated the expression of NMUR1.
3.4. Knockdown of NMUR1 abolished the protective effect of the miR-1275 antagomir on the cell injury in myocardial cells
In order to study the role of NMUR1 in the protective effect of miR-1275 inhibition on OGD/R damage, NMUR1 siRNA (si-NMUR1) was transfected into cardiomyocytes. As shown in ), the expression of NMUR1 mRNA and proteins was markedly inhibited by si-NMUR1 transfection. Further, we found NMUR1 expression was significantly declined in OGD/R stimulated cardiomyocytes, whereas the miR-1275 antagomir effectivity reversed the decline in NMUR1 that was induced by OGD/R stimulation ( and )). Next, si-NMUR1 enhanced the activity of LDH and CK in OGD/R treated cardiomyocytes, and abolished the inhibitory effect of the miR-1275 antagomir on the activity of LDH and CK ()). In addition, we found that si-NMUR1 promoted the production of ROS and cell apoptosis induced by OGD/R in cardiomyocytes, and suppressed the protective effect of miR-1275 antagomir on OGD/R treated cardiomyocytes ()).
Figure 4. Silence of NMUR1 reversed the effect of miR-1275 knockdown on cell injury in myocardial cell. (a, b) The expression of NMUR1 mRNA and proteins in cardiomyocytes was measured after transfection with si-NMUR1 (50 nM). **P < 0.01, compared with si-NC transfected group. (c–h) NMUR1 expression (c), LDH (e) and CK (f) activity, ROS levels (F) and cell apoptosis (g, h) in cardiomyocytes were detected after transfection with the miR-1275 antagomir, si-NMUR1 or co-transfection with the miR-1275 antagomir and si-NMUR1. Values are expressed as mean ± SEM, n = 3, *P < 0.05, **P < 0.01, compared with untreated group. #P < 0.05, compared with OGD/R-treated group; &P < 0.05, compared with OGD/R + anta-miR transfected group
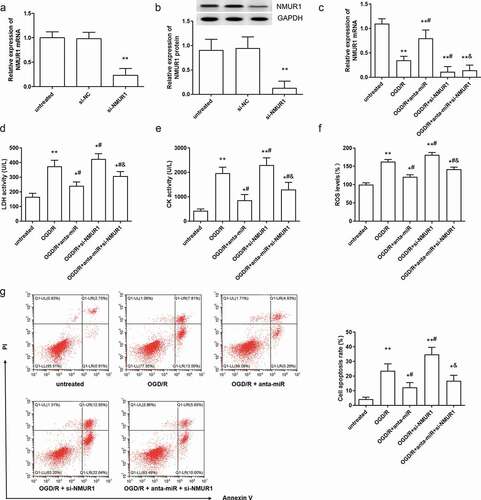
Figure 5. Silence of NMUR1 partly suppressed the inhibitory effect of miR-1275 knockdown on calcium overload in OGD/R in hESC-derived cardiomyocytes. (a, b). Intracellular Ca2+ concentration and SR Ca2+ leak in cardiomyocytes were examined. (c, d). The expressions of NMUR1, NCX1, RyR2, SERCA2a, PS16 PLN, and total-PLN (t-PLN) were examined by Western blot analysis. Values are expressed as mean ± SEM, n = 3, *P < 0.05, **P < 0.01, compared with untreated group. #P < 0.05, compared with OGD/R-treated group; &P < 0.05, compared with OGD/R + anta-miR transfected group
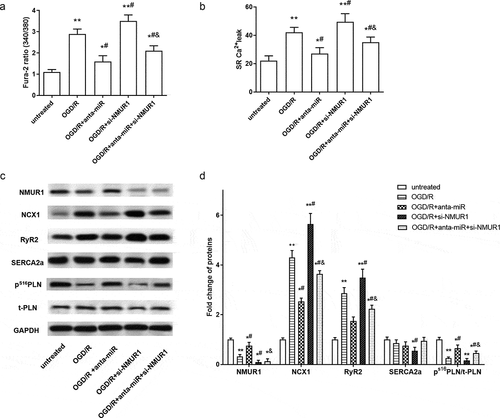
3.5. Knockdown of miR-1275 suppressed calcium overload in myocardial cells through upregulation of NMUR1
As shown in ), higher level of intracellular Ca2+ and SR Ca2+ leak was induced by NMUR1 inhibition in OGD/R-treated cardiomyocytes. At the same time, NMUR1 inhibition reversed the inhibitory effect of the miR-1275 antagomir on the intracellular Ca2+ concentration and SR Ca2+ leak in OGD/R-treated cardiomyocytes. Ca2+-cycle-related proteins were examined by Western blotting. The expressions of PS16 PLN (phospholamban, phosphorylated at S16) was downregulated in OGD/R-stimulated cardiomyocytes, and this decline was reversed by the miR-1275 antagomir, enhanced by NMUR1 silence ()). Meanwhile, NMUR1 downregulation partly inhibited the promoting effect of the miR-1275 antagomir on PS16 PLN ()). Inhibition of miR-1275 suppressed the increase of NCX1 (Na–Ca exchanger) and RyR2 (ryanodine receptor isoform 2) in OGD/R-stimulated cardiomyocytes. While inhibition of NMUR1 promoted the increase of NCX1 and RyR2 in OGD/R-stimulated cardiomyocytes. Importantly, si-NMUR1 abolished the inhibitory effect of the miR-1275 antagomir on NCX1 and RyR2 ()).
4. Discussion
It has been reported that OGD/R injury induces intracellular calcium overload, formation of oxygen radicals, and microvascular endothelial injury [Citation27]. Hence, hESC-derived cardiomyocytes treated by OGD/R injury were adopted in this study to mimic the pediatric CIRI. The differentiation of hESCs into cardiomyocytes was performed as previously reported and verified through testing the increasing expression of specific cardiomyocyte markers (GATA4, TNNT2, and MYL7) and the decline of hESC-specific markers (Oct-4 and Sox-2) [Citation26,Citation30]. In addition, the activities of LDH and CK, cell apoptosis, oxidative stress, the intracellular Ca2+ concentration, and the SR Ca2+ leak were increased in OGD/R-stimulated cardiomyocytes. LDH is a marker of cell injury, and it is found to be increased in myocardial infarction, acute or chronic hepatitis, and cell injury [Citation31,Citation32]. CK is an indicator of myocardial infarction, and it is found to be increased in myocardial infarction, viral myocarditis, and pericarditis [Citation33]. Apoptosis is a type of cardiomyocyte death [Citation34]. Ca2+ is necessary and indispensable during the excitation–contraction coupling process. The balance of the calcium cycle is fundamental to the proper functioning of hearts [Citation35]. When HF occurs, the regulatory mechanism of Ca2+ circulation is disordered, leading to intracellular Ca2+ overload and SR Ca2+ leak [Citation36]. Defective Ca2+ handling causes reversible, as well as irreversible myocardial injury and is a primary therapeutic target for cardioprotection. In our study, miR-1275 antagomir inhibited the increasing activity of LDH and CK, suppressed the increased cell apoptosis, oxidative stress, increased intracellular Ca2+ concentration, and SR Ca2+ leak in hESC-derived cardiomyocytes that were induced by OGD/R treatment. But we think the primary effect of miR-1275 in injured cardiomyocytes is regulating the calcium cycle, and consequently influenced the activity of LDH and CK, cell apoptosis, and ROS production. It has been reported that calcium cycle controlled the ROS production [Citation37] and participated in cell proliferation and death [Citation38]. This discussion has been added in revised MS. Hence, it was indicated that inhibition of miR-1275 protected the cardiomyocytes from OGD/R damage.
In the present study, our results demonstrated that miR-1275 directly targets NMUR1 and negatively regulates its expression. NMUR1, a receptor of neuromedin U, mainly participated in the regulation of energy homeostasis [Citation39]. It has been reported that NMUR1 is downregulated in the heart tissue of heart failure patients [Citation40]. This study proved that miR-1275 overexpression inhibited the expression of NMUR1, while miR-1275 inhibition promoted the expression of NMUR1. At the same time, silence of NMUR1 aggravated the cell injury induced by OGD/R in cardiomyocytes, and partly abolished the protective effect of the miR-1275 antagomir on myocardial OGD/R injury.
Furthermore, we studied the effect of miR-1275 and NMUR1 on oxidative stress and Ca2+ circulation. Our study found that NMUR1 knockdown weakened the inhibitory effect of miR-1275 antagomir on ROS production. It is widely considered that oxidative activated Ca2+ circulation contributes to cardiac dysfunction and apoptosis [Citation41]. Based on a previous report stating that activation of NMUR1 inhibits L-type high-voltage-gated Ca2+ channels in mouse hippocampal neurons [Citation42]. In this study, inhibition of NMUR1 exacerbated the increasing intracellular Ca2+ concentration and SR Ca2+ leak in OGD/R stimulated cardiomyocytes, reversed the inhibitory effect of the miR-1275 antagomir on the increasing Ca2+ overload in cardiomyocytes that induced by OGD/R. RyR2 (ryanodine receptor 2) is a Ca2+ release channel in the SR. It mediates the release of Ca2+ from the SR into the cytoplasm, and it is served as a therapeutic target in myocardial I/R injury [Citation23,Citation43,Citation44]. NCX1 is a Na–Ca exchanger, playing a key role in regulating intracellular Ca2+ concentration. It is upregulated in the progression of HF and contributes to myocardial hypertrophy, myocardial infarction, and heart failure [Citation45]. Our results are consistent with the previous suggestion that RyR2 and NCX1 were upregulated in the OGD/R injury myocardial model [Citation44]. The miR-1275 antagomir inhibited the expression of RyR2 and NCX1 in OGD/R-stimulated cardiomyocytes, and this inhibition was reversed by si-NMUR1. A previous study proved that knockdown of RyR2 reduces cell death and attenuating Ca2+ and ROS production, and it protected the cardiomyocytes from I/R injury [Citation46]. Consistent with previous reports, the expression of PS16 PLN was decreased in OGD/R-stimulated cardiomyocytes [Citation47]. Our study implied that miR-1275 antagomir promoted the expression of PS16 PLN in OGD/R-stimulated cardiomyocyte, while silencing NMUR1, partly inhibiting the promoting effect of the miR-1275 antagomir on PS16 PLN. These results suggested that inhibition of miR-1275 inhibited intracellular Ca2+ overload and SR Ca2+ through suppressing the expression of RyR2 and NCX1 and promoting the expression of PS16 PLN by directly targeting NMUR1. However, the underlying molecular mechanism of how NMUR1 functions in calcium cycle remain unclear. A previous study suggested that NMUR1 suppressed L-type Ca 2+ channel currents may though PI3K dependent protein kinase C epsilon pathway in mouse hippocampal neurons [Citation42]. Whether NMUR1 plays a role in calcium cycle in cardiomyocytes through the PI3K pathway still needs further study.
In summary, miR-1275 was upregulated in OGD/R-stimulated cardiomyocyte injury. Inhibition of miR-1275 suppressed the cell injury, cell apoptosis, oxidative stress, and disorder of Ca2+ circulation in cardiomyocytes that induced by OGD/R treatment, and this effect may be through the upregulation of NMUR1. Our study indicating that miR-1275 may be a therapeutic target for CIRI.
Supplemental Material
Download MS Word (536.6 KB)Data availability
The data used to support the findings of this study are included within the article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Vaage J, Valen G. Pathophysiology and mediators of ischemia-reperfusion injury with special reference to cardiac surgery: a review. Scand J Thorac Cardiovasc Surg Suppl. 1993;41:1–18.
- Zschirnt M, Jux C, Boenig H, et al. Neonatal myocardial infarction: substantial improvement of cardiac function after autologous bone marrow-derived cell therapy. Clin Res Cardiol. 2019;108:1309–1311.
- Solevåg AL, Schmölzer GM, Cheung PY. Right ventricular myocardial ischemia with arrhythmia in an asphyxiated newborn. Ajp Rep. 2016;6:e203–e205.
- Hausenloy DJ, Yellon DM. Targeting myocardial reperfusion injury — the search continues. N Engl J Med. 2015;373:1073–1075.
- Gao Y, Huang R, Chen R, et al. Ischemic postconditioning altered MicroRNAs in human valve replacement. J Surg Res. 2015;200:28-35.
- Jiang C, Liu X, Wang M, et al. High blood miR-802 is associated with poor prognosis in HCC patients by regulating DNA damage response 1 (REDD1)-mediated function of T cells. Oncol Res Featuring Preclinical Clin Cancer Ther. 2019;27:1025–1034.
- Montgomery RL, Hullinger TG, Semus HM, et al. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124:1537–1547.
- Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205.
- Biasucci LM, Cardillo MT. MicroRNA and myocardial infarction *. 2013;999–1001.
- Jing S, Yihua B, Xiangqing K, et al. miR-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury. Theranostics. 2017;7:664–676.
- Wang Y, Men M, Yang W, et al. MiR-31 downregulation protects against cardiac ischemia/reperfusion injury by targeting protein kinase C epsilon (PKCe) directly. Cell Physiol Biochem. 2015;36:179–190.
- Yao L, Lv X, Wang X. MicroRNA 26a inhibits HMGB1 expression and attenuates cardiac ischemia-reperfusion injury. J Pharmacol Sci. 2016;131:6–12.
- Park KM, Teoh JP, Wang Y, et al. Carvedilol-responsive microRNAs, miR-199a-3p and −214 protect cardiomyocytes from simulated ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2016;311:ajpheart.00807.2015.
- Fawzy IO, Hamza MT, Hosny KA, et al. miR‐1275: a single microRNA that targets the three IGF2‐mRNA‐binding proteins hindering tumor growth in hepatocellular carcinoma. FEBS Lett. 2016;589:2257–2265.
- Ma X, Yang X, Bao W, et al. Circular RNA circMAN2B2 facilitates lung cancer cell proliferation and invasion via miR-1275/FOXK1 axis. Biochem Biophys Res Commun. 2018;498:1009–1015.
- Kaiyu S, Zhe C, Cen Z, et al., Otorhinolaryngology DO. Effects of miR-1275 and its target gene HOXB5 on nasopharyngeal carcinoma cells. Med J Wuhan Univ. 2018;39:910–913.
- Pang L, You L, Ji C, et al. miR-1275 inhibits adipogenesis via ELK1 and its expression decreases in obese subjects. J Mol Endocrinol. 2016;57:33.
- Zhou YF, Fu ZY, Chen XH, et al. Tumor necrosis factor‑α and interleukin‑6 suppress microRNA‑1275 transcription in human adipocytes through nuclear factor‑κB. Mol Med Rep. 2017;16:5965-5971.
- Gu H, Chen L, Xue J, et al. Expression profile of maternal circulating microRNAs as non-invasive biomarkers for prenatal diagnosis of congenital heart defects. Biomed Pharmacother.2019;109:823-830.
- O’Brien JE, Kibiryeva N, Zhou XG, et al. Noncoding RNA expression in myocardium from infants with tetralogy of fallot. Circ Cardiovasc Genet. 2012;5:279–286.
- Ma Q, Zhao H, Tao Z, et al. MicroRNA-181c exacerbates brain injury in acute ischemic stroke. Aging Dis. 2016;7:705–714.
- Meng Y, Li W-Z, Shi Y-W, et al. Danshensu protects against ischemia/reperfusion injury and inhibits the apoptosis of H9c2 cells by reducing the calcium overload through the p-JNK-NF-κB-TRPC6 pathway. Int J Mol Med. 2016;37:258–266.
- Fauconnier J, Roberge S, Saint N, et al. Type 2 ryanodine receptor: a novel therapeutic target in myocardial ischemia/reperfusion. Pharmacol Ther. 2013;138:323–332.
- Ramachandran S, Lowenthal A, Ritner C, et al. Plasma microvesicle analysis identifies microRNA 129-5p as a biomarker of heart failure in univentricular heart disease. PLoS ONE. 2017;12:e0183624.
- Yoo H, Kim Y, Lee Y, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Investig. 2001;108:407–414.
- Zhang S, Zhang R, Wu F, et al. MicroRNA-208a regulates H9c2 cells simulated ischemia-reperfusion myocardial injury via targeting CHD9 through Notch/NF-kappa B signal pathways. Int Heart J. 2018;59:580–588.
- Zhu W, Liu F, Wang L, et al. pPolyHb protects myocardial H9C2 cells against ischemia-reperfusion injury by regulating the Pink1-Parkin-mediated mitochondrial autophagy pathway. Artif Cells Nanomed Biotechnol. 2019;47:1248–1255.
- Park S, Choe M, Yeo H, et al. Yes‐associated protein mediates human embryonic stem cell‐derived cardiomyocyte proliferation: involvement of epidermal growth factor receptor signaling. J Cell Physiol. 2018;233:7016–7025.
- Pereira L, Dan JB, Galice S, et al. β-Adrenergic induced SR Ca2+ leak is mediated by an Epac-NOS pathway. J Mol Cell Cardiol. 2017;108:8.
- Cao C, Li L, Li H, et al. Cyclic biaxial tensile strain promotes bone marrow-derived mesenchymal stem cells to differentiate into cardiomyocyte-like cells by miRNA-27a. Int J Biochem Biotechnol. 2018;99:125-132.
- Muchtar E, Dispenzieri A, Lacy MQ, et al. Elevation of serum lactate dehydrogenase in AL amyloidosis reflects tissue damage and is an adverse prognostic marker in patients not eligible for stem cell transplantation. Br J Haematol. 2017;178:888–895.
- Ghasemibasir H, Heidarpour M, Emami F, et al. Evaluation of total-LDH/heat resistant-LDH ratio in patients with acute myocardial infarction and unstable pectoral angina. J Shahrekord Univ Med Sci. 2009.
- Young GP, Gibler WB, Hedges JR, et al. Serial creatine kinase-MB results are a sensitive indicator of acute myocardial infarction in chest pain patients with nondiagnostic electrocardiograms: the second emergency medicine cardiac research group study. Acad Emerg Med. 2014;4:869–877.
- Ganguly R, Hasanally D, Stamenkovic A, et al. Alpha linolenic acid decreases apoptosis and oxidized phospholipids in cardiomyocytes during ischemia/reperfusion. Mol Cell Biochem. 2018;437:163–175.
- Santana LF, Gómez AM, Kranias EG, et al. Amount of calcium in the sarcoplasmic reticulum: influence on excitation-contraction coupling in heart muscle. Heart Vessels Suppl. 1997;12:44–49.
- Bers DM. Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu Rev Physiol. 2013;76:107.
- Bertero E, Maack C. Calcium signaling and reactive oxygen species in mitochondria. Circ Res. 2018;122:1460–1478.
- Humeau J, Bravo-San Pedro JM, Vitale I, et al. Calcium signaling and cell cycle: progression or death. Cell Calcium. 2018;70:3–15.
- Kaczmarek P, Malendowicz L, Pruszynska-Oszmalek E, et al. Neuromedin U receptor 1 expression in the rat endocrine pancreas and evidence suggesting neuromedin U suppressive effect on insulin secretion from isolated rat pancreatic islets. Int J Mol Med. 2006;18:951–955.
- Schiano C, Costa V, Aprile M, et al. Heart failure: pilot transcriptomic analysis of cardiac tissue by RNA-sequencing. Cardiol J. 2017;24:539–553.
- Nathan D, Roe JR. Oxidative activation of Ca2+/calmodulin-activated kinase II mediates ER stress-induced cardiac dysfunction and apoptosis. Am J Physiol Heart Circ Physiol. 2013;304:H828.
- Zhang Y, Jiang D, Zhang J, et al. Activation of neuromedin U type 1 receptor inhibits L-type Ca2+ channel currents via phosphatidylinositol 3-kinase-dependent protein kinase C epsilon pathway in mouse hippocampal neurons. Cell Signal. 2010;22:1660–1668.
- Respress JL, Oort RJV, Li N, et al. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012;110:1474.
- Carlo MND, Said M, Ling H, et al. CaMKII-dependent phosphorylation of cardiac ryanodine receptors regulates cell death in cardiac ischemia/reperfusion injury. J Mol Cell Cardiol. 2014;74:274–283.
- Menick DR, Renaud L, Buchholz A, et al. Regulation of Ncx1 gene expression in the normal and hypertrophic heart. Ann N Y Acad Sci. 2010;1099:195–203.
- Guo Z, Wang S, Jiao Q, et al. RNAi targeting ryanodine receptor 2 protects rat cardiomyocytes from injury caused by simulated ischemia-reperfusion. Biomed Pharmacother. 2010;64:184–190.
- Zhang DW, Bian ZP, Xu JD, et al. Astragaloside IV alleviates hypoxia/reoxygenation-induced neonatal rat cardiomyocyte injury via the protein kinase a pathway. Pharmacology. 2012;90:95–101.
