ABSTRACT
Glioma is one of the most aggressive malignancies in the central nervous system and the prognosis of glioma patients remains poor. In this study, we investigated the function of microRNA-30e-3p (miR-30e-3p) in glioma development and its regulatory role in drug-resistance to temozolomide (TMZ). We found that miR-30e-3p was downregulated in glioma tissues and cell lines. Ectopic expression of miR-30e-3p inhibited the growth of glioma cells and arrested cell cycle at G0/G1 phase. Canopy FGF signaling regulator 2 (CNPY2) was predicted as a direct target of miR-30e-3p by bioinformatics analysis. Luciferase reporter assay confirmed the interaction between miR-30e-3p and CNPY2. We also demonstrated that miR-30e-3p suppressed glioma xenograft tumor development invivo and the inhibition was abolished by CNPY2 overexpression. In addition, we showed that overexpression of miR-30e-3p enhanced the sensitivity of glioma cell to TMZ treatment. Glioma cells with miR-30e-3p overexpression had decreased cell proliferation and enhanced cell apoptosis upon TMZ treatment. Moreover, we revealed that miR-30e-3p modulated TMZ sensitivity of glioma cells via negatively regulating CNPY2. Taken together, our findings demonstrate that miR-30e-3p plays a critical role in glioma development and drug sensitivity to TMZ treatment via negatively regulating CNPY2 expression. The study suggests that miR-30e-3p/CNPY2 could be developed as a novel target to improve the glioma therapy.
Abbreviations: miR-30e-3p, microRNA-30e-3p; TMZ, temozolomide; CNPY2, canopy FGF signaling regulator 2; 3ʹ-UTR, 3ʹ untranslated region; NC, negative control.
KEYWORDS:
Introduction
Glioma is one of the most aggressive malignancies in the central nervous system and the prognosis of glioma patients remains poor [Citation1]. Glioma can be classified into different grades and the grade IV glioma is most aggressive one with less than one year survival [Citation2]. In addition, gliomas have high levels of inter-tumoral and intra-tumoral heterogeneity including tumorigenic cancer stem cell, which lead to high recurrence rates and treatment failure [Citation3]. Therefore, it is of great importance to understand the molecular mechanism underlying the development and progression of gliomas, and to develop novel therapeutic strategies for glioma treatment.
MicroRNAs (miRNAs) belong to a family of non-coding small RNAs participating post-transcriptional regulation of target gene expression [Citation4]. MiRNAs have been studied in various biological processes including tumorigenesis [Citation5]. Mounting studies reveal that miRNAs regulates tumor cell proliferation, migration, apoptosis and metastasis [Citation6]. In gliomas, dysregulated miRNAs have been demonstrated to play critical functions and act as tumor suppressor or oncogene in glioma development and progression [Citation7–9]. MicroRNA-30e-3p (miR-30e-3p) has been demonstrated to suppress clear cell renal cell carcinoma progression via negatively regulating Snail1 [Citation10]. In another study, Laura Gramantieri et al. showed that miR-30e-3p modulated MDM2/TP53 axis and predicted the development of Sorafenib resistance in hepatocellular carcinoma patients [Citation11]. However, the function of miR-30e-3p in glioma development and progression remains unknown.
Canopy fibroblast growth factor signaling regulator 2 (CNPY2), a member of canopy family, is widely expressed in multiple tissues and function as an angiogenic growth factor [Citation12]. Studies have shown that CNPY2 participates in regulating cell proliferation, migration and tissue revascularization in multiple malignancies [Citation13,Citation14]. In colorectal cancer, serum CNPY2 level could be explored as a novel biomarker for early diagnosis [Citation15]. CNPY2 also mediated the drug resistance to cisplatin treatment in human non-small cell lung cancer via activating NF-κB signaling pathway [Citation16]. Nevertheless, how CNPY2 is regulated and its role in glioma is not clear.
Here, we found that miR-30e-3p was low-expressed in glioma tissues and cell lines. Overexpression of miR-30e-3p suppressed glioma cell proliferation in vitro and glioma xenograft tumor development in vivo. CNPY2 was identified as a direct target of miR-30e-3p and miR-30e-3p exerted its regulatory function in glioma development via negatively regulating CNPY2 expression. Furthermore, we demonstrated that miR-30e-3p/CNPY2 modulated the TMZ sensitivity in glioma treatment. Thus, our findings suggest miR-30e-3p plays a critical role in glioma development and drug sensitivity to TMZ treatment via negatively regulating CNPY2 expression, which could be utilized to develop new therapeutic strategies for glioma treatment.
Materials and methods
Patient specimen
Clinical samples were obtained from glioma patients at the First Affiliated Hospital of Xi’an Jiaotong University. This study was reviewed and approved (2019–231) by the Institutional Ethics Committee of the the First Affiliated Hospital of Xi’an Jiaotong University and conducted following the Helsinki Declaration. All subjects signed the written informed consent. The diagnosis of glioma was confirmed by two independent histopathologists. All the tissue samples were snap-frozen and stored in liquid nitrogen until use.
Culture and cell transfection
The human glioma cell lines (A172, U87, U251, LN229, and LN18) and normal human astrocytes (NHA) were purchased from American Type Culture Collection (ATCC, USA). Cryopreserved hFOB1.19 cells were recovered from liquid nitrogen using an AccuVital cell recovery kit (AccuRef Scientific, Xi’an, China) and cultured in DMEM supplied with 10% fatal bovine serum (GIBCO, USA), 1% penicillin and streptomycin. Human-specific miR-30e-3p mimic, miR-30e-3p inhibitor, and relative negative control (NC) were commercial synthesized (Genebio, China). CNPY2 open-reading frame was amplified by PCR and cloned into pcDNA3.1 vector to construct pcDNA3.1-CNPY2 vector. Cells were transfected by using FuGene HD reagent (Roche, Switzerland) according to the instruction manual.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
RNA isolation, cDNA synthesis and qRT-PCR were conducted as previously described [Citation1]. The 2-ΔΔCt methods were used to calculate the relative expression. The primer sequences used in this study were listed below: miR-30e-3p, forward 5ʹ-ACGCTTTCAGTCGGATGTTTACAGC, reverse GTGCGTGTCGTGGAGTCG; CNPY2, forward 5ʹ-CAAACTACAGGTCCCAGGATAG-3ʹ, reverse 5ʹ-CACAGGAAACTACATCTCCCA-3ʹ; COLEC12, forward 5ʹ-GGTGACCAAACTGGGAAGAA, reverse 5ʹ-TATACGCCTGGAGGGTTTTG; CDKN1B, forward 5ʹ-ATGTCAAACGTGCGAGTGTC-3ʹ, reverse, 5ʹ-TCTCTGCAGTGCTTCTCCAA-3ʹ; SLC25A33, forward, 5ʹ-GGACCATTAGTGGAGCTGGA-3ʹ, reverse, 5ʹ-TTGCTCTTTGGCTTTGGAGT-3ʹ; WDR44, forward, 5ʹ-CAGGGGTATGCAGTGGAACT-3ʹ, reverse, 5ʹ-GCAAAGGCATTCTCTTCGAG-3ʹ; HIRA, forward, 5ʹ-GCCTGAAGTTCACCGAAGAG-3ʹ, reverse, 5ʹ-CTGGTTGGCCTCGTTATTGT-3ʹ; PTEN, forward, 5ʹ-TAAAGCTGGAAAGGGCAGAA-3ʹ, reverse, 5ʹ-ACTGGACTCCGAGAAGCGTA-3ʹ.
Western blot
Western blot was conducted following standard protocol. Total protein was prepared from tissues or cultured cells using RIPA buffer (AccuRef Scientific). Samples containing equal amounts of protein (20 μg for each lane) were mixed with a loading buffer (AccuRef Scientific) and separated by 10% SDS-PAGE followed by transferring to a PVDF membrane. The primary antibodies used were listed below: anti-CNPY2 antibody (cat.no. ab233136). To control the sample loading, the membranes were stripped with a stripping buffer (AccuRef Scientific), and re-probed with β-actin (cat.no. ab8226) antibody. All the primary antibodies were purchased from Abcam (Cambridge, MA). The secondary horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (cat. no HS101, TransGen Biotech, Inc.) or anti-mouse IgG (cat. no. HS201, TransGen Biotech, Inc.) antibodies were used. Protein bands were visualized using the enhanced chemiluminescence (ECL) kit (Pierece) and quantified using Image-pro Plus 5.0 software (NIH, USA).
CCK-8 and colony formation assay
Cell proliferation was analyzed by cell counting kit-8 (CCK-8) following the manufacturer’s protocol (AccuRef Scientific). For colony formation, 500 transfected cells were seeded into 6-well plates and cultured for 2 weeks. Then cells were fixed and the colonies were visualized via crystal violet staining and counted under a microscope.
Luciferase assay
The WT or mutated 3ʹ-UTR of CNPY2 with miR-30e-3p binding site was amplified and sub-cloned into luciferase reporter vectors. Then miR-30e-3p mimic and luciferase reporter vectors were co-transfected into A172 cells using FuGene HD (Roche, Switzerland). After incubation for 48 hours, the luciferase activities were tested using the dual-luciferase reporter assay system (Promega, USA).
Immunochemistry (IHC)
IHC was conducted as described previously [Citation1]. The primary antibodies used for IHC were listed below: anti-Ki-67 antibody (cat.no. ab15580), anti-CNPY2 antibody (cat.no. ab233136) were all purchased from Abcam (Cambridge, MA). The secondary horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (cat. no HS101, TransGen Biotech, Inc.) was used. DAB (cat. no. 1005) was purchased from Makewonderbio (Beijing, China).
Flow cytometry
Cell cycle and cell apoptosis were analyzed by flow cytometry. Briefly, transfected cells were fixed with 70% ethanol and then incubated with 50 μg/ml propidium iodide (PI, Sigma-Aldrich, USA) and 10 μg/ml RNase A for 30 min at room temperature. Cells were suspended in PBS and analyzed using FACScalibur (BD Bioscience, USA). Cell apoptosis was analyzed by Annexin V-FITC/PI double staining using a cell apoptosis kit (BD Bioscience, USA). The flow cytometry data was analyzed using CellQuest software (BD Bioscience, USA).
Xenograft tumor model
Xenograft tumor model was established by inoculating U87 cells (1 × 107) stably transfected with miR-30e-3p mimic, miR-30e-3p inhibitor, miR-30e-3p mimic+pcDNA3.1-CNPY2, or NC into the flanks of nude mice (male, 5 weeks old). Tumor growth was measured at indicated times. 25 days later, mice were sacrificed and tumor weights were evaluated. The animal experiment was reviewed and approved (XJTULAC20190113) by the Animal Care and Use committee of the First Affiliated Hospital of Xi’an Jiaotong University.
Statistical analysis
Statistical analyses were performed using GraphPad Prism V6 (Prism, USA). The data were expressed as mean ± standard deviation. Unpaired student t test and one-way ANOVA followed by Tukey’s comparisons were used where necessary. Differences were statistically significant with a P value < 0.05.
Results
MiR-30e-3p is downregulated in glioma tissues and cell lines
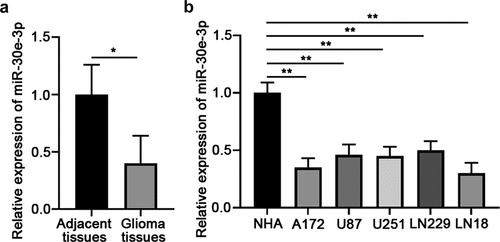
To study the function of miR-30e-3p in glioma development and progression, we first examined the expression of miR-30e-3p in glioma tissues and normal brain tissues. As shown in , miR-30e-3p expression was significantly lower in glioma tissues compared with adjacent normal tissues. In addition, we found that in comparison with the miR-30e-3p expression in normal human astrocytes (NHA), the expression of miR-30e-3p was markedly decreased in glioma cell lines (A172, U87, U251, LN229 and LN18). Thus, miR-30e-3p was low expressed in glioma, which might play an important role in glioma development and progression.
Figure 1. MiR-30e-3p is low-expressed in glioma tissues and cell lines. (a) Relative expression level of miR-30e-3p in human glioma tissues and adjacent non-tumor tissues was detected by qRT-PCR. (b) Relative expression level of miR-30e-3p in glioma cell lines (A172, U87, U251, LN229 and LN18) and normal human astrocytes (NHAs) was detected by qRT-PCR. *p < 0.05, ** p < 0.01. n = 5 for each group
MiR-30e-3p negatively regulates the growth of glioma cell
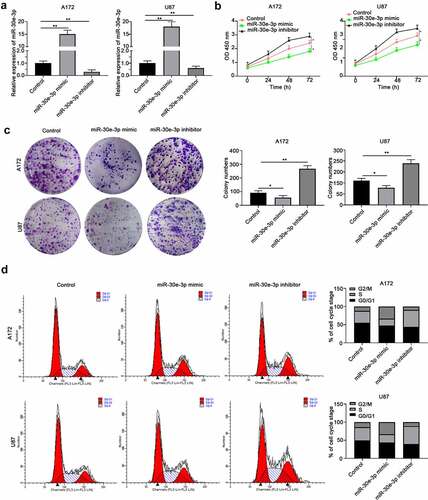
In order to evaluate the function of miR-30e-3p in glioma, miR-30e-3p mimic or inhibitor was transfected into A172 or U87 cells to overexpressing miR-30e-3p or inhibiting miR-30e-3p expression (). CCK-8 assay revealed that miR-30e-3p mimic inhibited glioma cell proliferation while miR-30e-3p inhibitor enhanced cell proliferation of A172 or U87 cells (). Consistently, colony formation experiment showed that A172 or U87 cells transfected with miR-30e-3p had less colonies compared with the control group, and inhibition of miR-30e-3p promoted colony formation of glioma cells (). Moreover, cell cycle analysis demonstrated that overexpression of miR-30e-3p arrested glioma cells at G0/G1 phase while miR-30e-3p inhibitor promoted cells entering G2/M and S phases (). Collectively, these findings suggest that miR-30e-3p negatively regulates the growth of glioma cell.
Figure 2. MiR-30e-3p negatively regulates the growth of glioma cell. A172 or U87 cells were transfected with miR-30e-3p mimic, inhibitor or negative control. (a) Relative expression of miR-30e-3p in A172 or U87 cells was analyzed by qRT-PCR 48 hours post transfection. (b) CCK-8 assay was performed to evaluate the cell proliferation. (c) Colony formation assay was performed to evaluate the cell colony formation. (d) Cell cycle analysis was performed to examine the different phases of cell cycle of A172 or U87 cells. * p < 0.05, ** p < 0.01. n = 5 for each group
CNPY2 is a direct target of miR-30e-3p in GBM cells
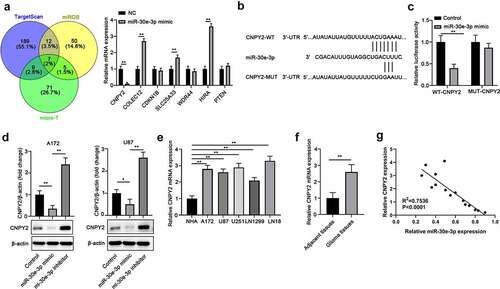
Bioinformatics analysis was performed to search for the potential targets regulated by miR-30e-3p using TargetScan, miRDB, and micro-T tools and seven genes were among the common targets predicted by all three databases (). CNPY2 was the most down-regulated gene by miR-30e-3p overexpression in A172 cells (). Luciferase reporter assay demonstrated that miR-30e-3p mimic significantly suppressed the luciferase activity of reporter containing WT 3ʹ-UTR of CNPY2, but not mutated 3ʹ-UTR of CNPY2 in A172 cells (). Furthermore, we found that overexpression of miR-30e-3p inhibited CNPY2 protein expression whereas miR-30e-3p inhibitor enhanced CNPY2 expression in A172 or U87 cells (). In contrast to the low expression of miR-30e-3p in glioma cells and tissues, CNPY2 expression was upregulated in glioma cell lines and tissues (). Moreover, Pearson analysis indicated that the expression of CNPY2 was negatively correlated with miR-30e-3p expression in glioma tissues ().
Figure 3. CNPY2 is a direct target of miR-30e-3p in GBM cells. (a) Bioinformatics analysis was performed using TargetScan, miRDB, and micro-T to predict the potential targets of miR-30e-3p. The expression of underlying targets was detected in cells after transfected with in A172 cells. (b) The putative base pairing between miR-30e-3p and CNPY2 was shown. (c) A172 cells were transfected with miR-30e-3p mimic or NC, and luciferase reporter vector containing WT or mutated 3ʹ-UTR of CNPY2. The relative luciferase activity was analyzed 48 hours post transfection. (d) A172 or U87 cells were transfected with miR-30e-3p mimic, inhibitor or negative control. The protein expression of CNPY2 was analyzed. (e) The mRNA level of CNPY2 was analyzed in glioma cell lines and NHA cells by qRT-PCR. (f) The mRNA levels of CNPY2 in glioma tissues and normal tissues were analyzed by qRT-PCR. (g) Pearson correlation analysis of CNPY2 and miR-30e-3p expression in glioma tissues. * p < 0.05, ** p < 0.01. n = 5 for each group
MiR-30e-3p inhibits glioma development via negatively regulating CNPY2 in vivo
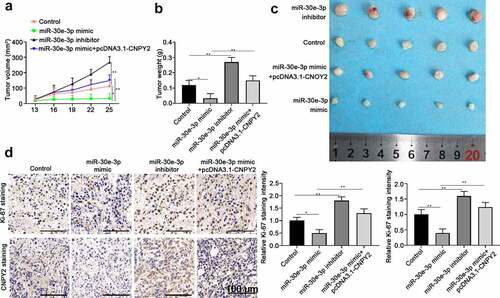
The functional relationship of miR-30e-3p and CNPY2 was further studied invivo using glioma xenograft tumor model. U87 cells were transfected with NC, miR-30e-3p mimic, miR-30e-3p inhibitor, or miR-30e-3p mimic + CNPY2 overexpression vector. The results showed that miR-30e-3p overexpression suppressed glioma xenograft development and inhibition of miR-30e-3p promoted tumor development of glioma compared with that of control group (). However, overexpression of CNPY2 abolished the tumor inhibitory effect of miR-30e-3p overexpression (). The expression of CNPY2 and proliferation marker Ki-67 was examined by immunohistochemical staining using xenograft tumor sections and the results confirmed that miR-30e-3p overexpression negatively regulated CNPY2 expression and suppressed cell proliferation (). Overexpression of CNPY2 abrogated the regulatory function of miR-30e-3p, showing the enhanced expression of CNPY2 and Ki-67 in tumor tissues ().
Figure 4. MiR-30e-3p inhibits glioma development via negatively regulating CNPY2 in vivo. U87 cells were transfected with negative control, miR-30e-3p mimic, miR-30e-3p inhibitor, or miR-30e-3p mimic + CNPY2 overexpression vector. 5 × 106 cells were inoculated into nude mice to establish the xenograft tumor model. (a) The tumor growth was assessed at indicated time points. (b, c) Representative tumor photos were shown and tumor weight were analyzed after xenograft tumor extraction. (d) The expression of Ki-67 and CNPY2 in tumor tissues was analyzed by immunohistochemical staining. * p < 0.05, ** p < 0.01. n = 5 for each group
MiR-30e-3p overexpression sensitizes glioma cell to TMZ treatment
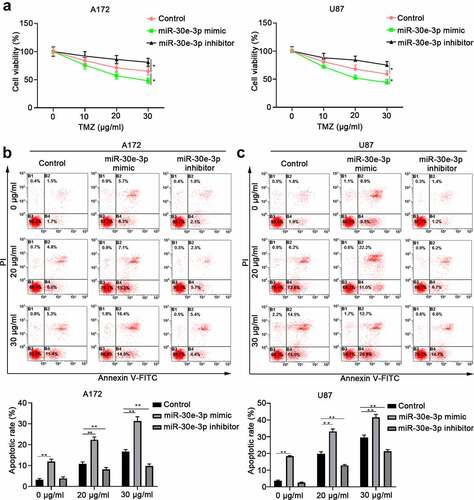
TMZ has been used as first-line treatment for glioma but drug resistant accounts for glioma treatment failure [Citation17]. A172 or U87 cells were transfected with miR-30e-3p mimic, miR-30e-3p inhibitor or NC, and then treated with different concentrations of TMZ. As shown in , miR-30e-3p overexpression significantly improved the sensitivity to TMZ treatment in A172 or U87 cells with much lower cell viability. In addition, we demonstrated that overexpression of miR-30e-3p enhanced glioma cell apoptosis while inhibition of miR-30e-3p suppressed glioma cell apoptosis upon TMZ treatment (). The findings suggest that miR-30e-3p might sensitize glioma cell to TMZ treatment.
Figure 5. MiR-30e-3p overexpression sensitizes glioma cell to TMZ treatment. A172 or U87 cells were transfected with miR-30e-3p mimic, miR-30e-3p inhibitor or negative control, and then treated with different concentrations of TMZ for 48 hours. (a) Cell proliferation was evaluated by CCK-8 assay. (b, c) Cell apoptosis of A172 or U87 cells was evaluated by Annexin V-FITC/PI dual staining. * p < 0.05, ** p < 0.01. n = 5 for each group
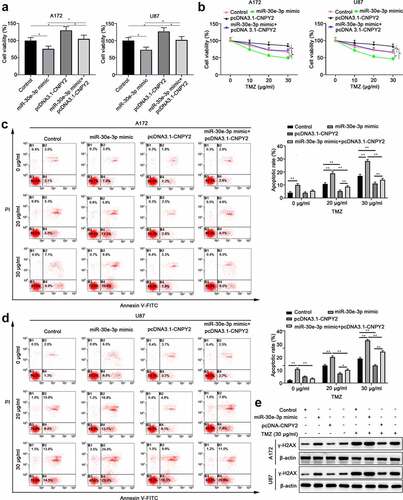
MiR-30e-3p modulates TMZ sensitivity of glioma cells via negatively regulating CNPY2
In order to investigate whether miR-30e-ep promotes TMZ sensitivity of glioma cells via regulating CNPY2, A172 or U87 cells were transfected with control vector, miR-30e-3p mimic, or miR-30e-3p + pcDNA3.1-CNPY2. Overexpression of miR-30e-3p suppressed cell proliferation and CNPY2 overexpression abolished the inhibitory of cell proliferation mediated by miR-30e-3p (). In addition, whereas miR-30e-3p mimic enhanced TMZ sensitivity of glioma cells, overexpression CNPY2 together with miR-30e-3p abrogated the TMZ sensitivity in A172 or U87 cells (). MiR-30e-3p mimic transfection enhanced cell apoptosis of glioma cells upon TMZ treatment. However, overexpression of CNPY2 dampened the cell apoptosis mediated by miR-30e-3p overexpression (). In addition, we have detected the expression of γ-H2AX in cells treated with miR-30e-3p mimic transfection or TMZ treatment to explore the DNA damage in glioma cells. The results indicated that miR-30e-3p mimic could significantly increase the γ-H2AX expression and CNPY2 overexpression could partially reverse the upregulation of γ-H2AX. TMZ treatment could obviously enhance the upregulation of miR-30e-3p induced by miR-30e-3p mimic, but CNPY2 overexpression could partially reverse the upregulation of γ-H2AX induced by miR-30e-3p mimic and TMZ treatment (). Collectively, our findings suggest that miR-30e-3p modulates TMZ sensitivity of glioma cells via negatively regulating CNPY2.
Figure 6. MiR-30e-3p modulates TMZ sensitivity of glioma cells via negatively regulating CNPY2. A172 or U87 cells were transfected with control, miR-30e-3p mimic, miR-30e-3p mimic+ pcDNA3.1, and miR-30e-3p mimic+ pcDNA3.1-CNPY2. (a) Cell proliferation was evaluated by CCK-8 assay after 48 hours. (b) Cells were treated with TMZ for 48 hours and the cell viability was analyzed. (c, d) Cell apoptosis of A172 or U87 cells treated with TMZ was evaluated by Annexin V-FITC/PI dual staining. (e) Expression of γ-H2AX in cells determined by western blot. * p < 0.05, ** p < 0.01. n = 5 for each group
Discussion
Dysregulated miRNA expression has been reported in glioma development and progression and miRNAs function as tumor suppressors or oncogenes [Citation18]. However, the detailed role and function of miRNAs in glioma have not been sufficient understood. In this study, we revealed a low-expressed miR-30e-3p in glioma tissues and cell lines. The functional assay demonstrated that overexpression of miR-30e-3p suppressed glioma cell growth and xenograft glioma development via negatively regulating CNPY2. Moreover, the miR-30e-3p/CNPY2 axis also modulated the TMZ sensitivity of glioma cells, which might be involved in drug-resistance of TMZ treatment in glioma patients.
MiR-30e-3p has been reported to be downregulated in cardiovascular diseases after coronary microembolization and regulate myocardial autophagy [Citation19]. In ovarian cancer, miR-30e-3p could negatively regulate LAMA4 expression and enhance the ovarian cancer development [Citation20]. Intriguingly, lncRNA MEG3 sponged miR-30e-3p expression in ovarian cancer [Citation20]. A recent study found that low expression of miR-30e-3p in hepatocellular carcinoma predicted sorafenib resistance and miR-30e-3p regulated MDM2/TP53 axis [Citation11]. These studies suggest a critical role of miR-30e-3p in tumorigenesis and drug-resistance. Here we showed that miR-30e-3p negatively regulated CNPY2 expression in gliomas and miR-30e-3p/CNPY2 mediated the chemo-sensitivity to TMZ treatment in gliomas.
CNPY2 has been widely studied in angiogenesis in various tissues [Citation12,Citation21]. Jianhong Peng etal. identified that CNPY2 represented as a valuable diagnosis biomarker and prognostic indicator for colorectal cancer patient, but the oncologic function of CNPY2 remains unknown [Citation22]. The oncologic role of CNPY2 has been investigated in renal cell carcinoma [Citation23], non-small cell lung cancer [Citation16,Citation24], and prostate cancer [Citation14]. CNPY2 was found to be downregulated in lung adenocarcinoma and miR-30a-3p directly targeted CNPY2 [Citation25]. In this study, we expanded the knowledge of CNPY2 function to glioma development and progression. CNPY2 enhanced glioma cell proliferation and colony formation, while overexpression of CNPY2 conferred the anti-apoptosis of glioma cells to TMZ treatment. Thus, targeting CNPY2 could be a potential promising therapeutic strategy in glioma treatment. TMZ resistance remains a great challenge for glioma treatment and targeting dysregulated miRNAs could improve the TMZ resistance in glioma therapy [Citation26, 27]. We demonstrated that miR-30e-3p overexpression could enhance the TMZ sensitivity of glioma cells via inhibiting the expression of CNPY2.
Though we have revealed the function role of miR-30e-3p/CNPY2 in glioma development and TMZ-resistance, a few limitations existed in the study. First, the prognosis values of miR-30e-3p/CNPY2 in glioma have not been extensively evaluated. Second, due to the multiple-to-multiple regulation between miRNAs and mRNAs, we couldn’t exclude the other regulatory mechanisms involved in regulating miR-30e-3p/CNPY2 expression [Citation26].
In conclusion, our results suggest that miR-30e-3p/CNPY2 regulates glioma progression and sensitivity to TMZ treatment, which might provide a new diagnosis and therapeutic target for glioma treatment.
Authors’ contributions
Tuo Wang, Yuan Qiao, Bo Cui evaluated the results. Ke Gao performed the experiment. Ke Gao, Tuo Wang, Yuan Qiao, Bo Cui participated in the design of the study.
Ethics approval and consent to participate
The ethics committee of the First Affiliated Hospital of Xi’an Jiaotong University approved the study. The investigation conforms to the principles outlined in the Declaration of Helsinki and written informed consent was obtained from all participants.
Availability of data and material
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Acknowledgments
The authors are very grateful for the generous assistance from Key Research and Development Program of Shaanxi (Grant No. 2019SF-104) and NSFC (Grant No. 81602210, 81702487).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Y L, R S, J Z, et al. miR-506 inhibits the proliferation and invasion by targeting IGF2BP1 in glioblastoma. Am J Transl Res. 2015;7:2007–2014.
- Chen R, Smith-Cohn M, Cohen AL, et al. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284–297.
- Reardon DA, Wen PY. Glioma in 2014: unravelling tumour heterogeneity-implications for therapy. Nat Rev Clin Oncol. 2015;12(2):69–70.
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233.
- Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016;1(1):15004.
- Manikandan J, Aarthi JJ, Kumar SD, et al. Oncomirs: the potential role of non-coding microRNAs in understanding cancer. Bioinformation. 2008;2(8):330–334.
- Chen W, Qi J, Bao G, et al. Emerging role of microRNA-27a in human malignant glioma cell survival via targeting of prohibitin. Mol Med Rep. 2015;12(1):1515–1523.
- Banelli B, Forlani A, Allemanni G, et al. MicroRNA in glioblastoma: An overview. Int J Genomics. 2017;(2017):7639084.
- Henriksen M, Johnsen KB, Andersen HH, et al. MicroRNA expression signatures determine prognosis and survival in glioblastoma multiforme–a systematic overview. Mol Neurobiol. 2014;50(3):896–913.
- Wang D, Zhu C, Zhang Y, et al. MicroRNA-30e-3p inhibits cell invasion and migration in clear cell renal cell carcinoma by targeting Snail1. Oncol Lett. 2017;13(4):2053–2058.
- Gramantieri L, Pollutri D, Gagliardi M, et al. MiR-30e-3p influences tumor phenotype through MDM2/TP53 axis and predicts sorafenib resistance in hepatocellular carcinoma. Cancer Res. 2020;80(8):1720–1734.
- Hatta K, Guo J, Ludke A, et al. Expression of CNPY2 in mouse tissues: quantification and localization. PLoS One. 2014;9(11):e111370.
- Yan P, Gong H, Zhai X, et al. Decreasing CNPY2 expression diminishes colorectal tumor growth and development through activation of p53 pathway. Am J Pathol. 2016;186(4):1015–1024.
- Ito S, Ueno A, Ueda T, et al. CNPY2 inhibits MYLIP-mediated AR protein degradation in prostate cancer cells. Oncotarget. 2018;9(25):17645–17655.
- Peng J, Ou Q, Pan Z, et al. Serum CNPY2 isoform 2 represents a novel biomarker for early detection of colorectal cancer. Aging (Albany NY). 2018;10(8):1921–1931.
- Yu D, Qin Y, Jun-Qiang L, et al. CNPY2 enhances resistance to apoptosis induced by cisplatin via activation of NF-kappaB pathway in human non-small cell lung cancer. Biomed Pharmacother. 2018;103:1658–1663.
- Zhang J, Stevens MF, Laughton CA, et al. Acquired resistance to temozolomide in glioma cell lines: molecular mechanisms and potential translational applications. Oncology. 2010;78(2):103–114.
- Wang BC, Ma J. Role of MicroRNAs in malignant glioma. Chin Med J (Engl). 2015;128(9):1238–1244.
- Wang XT, Wu XD, Lu YX, et al. Potential involvement of MiR-30e-3p in myocardial injury induced by coronary microembolization via autophagy activation. Cell Physiol Biochem. 2017;44(5):1995–2004.
- Liu Y, Xu Y, Ding L, et al. LncRNA MEG3 suppressed the progression of ovarian cancer via sponging miR-30e-3p and regulating LAMA4 expression. Cancer Cell Int. 2020;20(1):181.
- Guo J, Zhang Y, Mihic A, et al. A secreted protein (Canopy 2, CNPY2) enhances angiogenesis and promotes smooth muscle cell migration and proliferation. Cardiovasc Res. 2015;105(3):383–393.
- Peng J, Ou Q, Guo J, et al. Expression of a novel CNPY2 isoform in colorectal cancer and its association with oncologic prognosis. Aging (Albany NY). 2017;9(11):2334–2351.
- Taniguchi H, Ito S, Ueda T, et al. CNPY2 promoted the proliferation of renal cell carcinoma cells and increased the expression of TP53. Biochem Biophys Res Commun. 2017;485(2):267–271.
- Dou Y, Lei JQ, Guo SL, et al. The CNPY2 enhances epithelial-mesenchymal transition via activating the AKT/GSK3beta pathway in non-small cell lung cancer. Cell Biol Int. 2018;42(8):959–964.
- Wang H, Kanmangne D, Li R, et al. miR30a3p suppresses the proliferation and migration of lung adenocarcinoma cells by downregulating CNPY2. Oncol Rep. 2020;43:646–654.
- Hashimoto Y, Akiyama Y, Yuasa Y. Multiple-to-multiple relationships between microRNAs and target genes in gastric cancer. PLoS One. 2013;8(5):e62589.
