ABSTRACT
Mitosis is a key process in development and remains critical to ensure homeostasis in adult tissues. Besides its primary role in generating two new cells, cell division involves deep structural and molecular changes that might have additional effects on cell and tissue fate and shape. Specific quantitative and qualitative regulation of mitosis has been observed in multiple morphogenetic events in different embryo models. For instance, during mouse embryo gastrulation, the portion of epithelium that undergoes epithelial to mesenchymal transition, where a static epithelial cell become mesenchymal and motile, has a higher mitotic index and a distinct localization of mitotic rounding, compared to the rest of the tissue. Here we explore the potential mechanisms through which mitosis may favor tissue reorganization in various models. Notably, we discuss the mechanical impact of cell rounding on the cell and its environment, and the modification of tissue physical parameters through changes in cell-cell and cell-matrix adhesion.
Mitosis, guardian of epithelial homeostasis
Cell division must be tightly regulated during embryogenesis [Citation1,Citation2] and throughout life to maintain homeostasis or allow growth and regeneration [Citation3,Citation4]. Dysregulation of cell mitosis rate can lead to developmental defects [Citation5,Citation6] and is a hallmark of tumorigenesis [Citation7].
Epithelia separate biological compartments, and thereby constitute the major building block for patterning and shaping organs in metazoan organisms [Citation3,Citation8,Citation9,Citation10]. They are either simple (one layer), stratified (several layers), or pseudostratified (one single layer of densely packed elongated cells whose nuclei are distributed along the apical-basal axis, leading to a multi-layered appearance). Pseudostratified epithelia are often transient developmental structures characterized by high proliferation and dynamic rearrangements. In all types, juxtaposed cells tightly adhere to one another and the extracellular matrix, and display apical-basal polarization [Citation11,Citation12]. Polarity proteins, notably the partitioning-defective (PAR) complex, composed of PAR3, PAR6 and atypical protein kinase C (aPKC), localize apically in association with tight junction proteins and the Crumbs complex (CRB, PALS1, PATJ). PAR3 is the scaffold protein that initiates the formation of the PAR complex and sustains the maturation and maintenance of tight junctions [Citation13] (). Classically, dividing cells in epithelia retain apical-basal polarity and remain adhesive; however, this depends on the geometry of the epithelium and it is not a universal feature [Citation14]. For instance, in the Drosophila wing disc, adherens junctions are transiently downregulated during mitosis, via the activation of Rho GTPases leading to endocytosis of E-cadherin [Citation15]. Components of cell-matrix junctions can also be lost during division [Citation16] (). Likewise, cell cycle-coupled temporary loss of apical-basal polarity has been observed, notably in Drosophila follicular epithelium or Nematostella embryonic epithelium [Citation17,Citation18].
Figure 1. Architecture of an epithelial cell in interphase and entering mitosis. (a) representation of a cell in interphase in a pseudostratified epithelum. Cells are oriented along the apical-based axis. The apical pole (top) is exposed to the lumen while the basal pole (bottom) is in contact with the basement membrane, notably through integrins. Polarity complexes ensure the establishment and maintenance of polarity and cell-cell adhesion. (b) cell in prophase/prometaphase. When cells enter mitosis, actin cytoskeleton reorganization and osmotic swelling lead to cell rounding, which generates pushing forces towards the environment. Adherences (unctionsCell-cell, as well as cell-matrix, adhesions are transiently downregulated
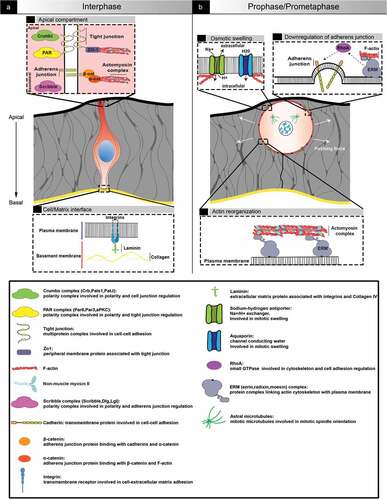
Epithelial cells are exposed to chemical and mechanical signals from each other and the environment. They integrate the information and ensure equilibrium between cell death and cell division in order to preserve epithelial function as a barrier while carrying out morphological changes [Citation3,Citation19]. Epithelial cells can adjust the forces generated by their own cytoskeleton network to forces exerted by neighboring cells and the extracellular matrix. For instance, adherens junctions are directly connected to the actomyosin network [Citation9,Citation20], which is in turn linked to the nucleoskeleton [Citation21]. Therefore, cell proliferation is influenced by cell density, but also by tissue shape and geometry. The evolutionary conserved Hippo kinase cascade connects environmental mechanical cues to cell division regulation via the retention or phosphorylation of the transcription co-activator YAP [Citation22]. The cation channel Piezo1 is also a mechanosensor involved in epithelial regulation. It can trigger cell proliferation or cell extrusion depending on its subcellular localization [Citation23,Citation24]. Mechanical signals are thus integrated molecularly through mechanochemical pathways and transferred across the cell to impact its architecture and by extension the dynamic of the entire epithelium.
Cell division represents far more than just an increase in cell number. Here, we aim to reflect on the relationship between mitosis and epithelial plasticity: mechanical changes influence cell mitosis rate, and reciprocally cell shape changes associated with division have an impact on tissue architecture [Citation25]. We will focus on the relationship between the cell cycle and epithelial-mesenchymal transition (EMT), using mouse embryo gastrulation as primary model.
Gastrulation
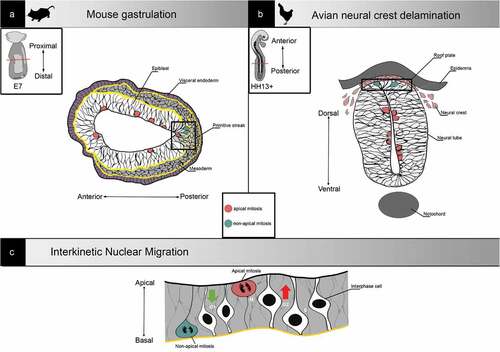
Gastrulation is an evolutionary conserved program that gives rise to the germ layers required for organogenesis [Citation5]. In the mouse embryo, this transition occurs in the epiblast, a pseudostratified epithelium. At embryonic day (E) 6, the posterior epiblast forms an intermediate structure, the primitive streak, where cells delaminating to become mesoderm or endoderm adopt a “bottle shape”, characterized by apical shrinkage and basal translocation of the nucleus () [Citation26]. In the primitive streak, the apical protein Crumbs2 shows an anisotropic pattern with higher expression in apically constricted cells. Crumbs2 and Myosin IIb levels are inversely correlated, which suggests that cells with high apical Myosin IIb push Crumbs2 positive cells out of the epithelium [Citation27]. Prospective mesoderm cells undergo a full EMT, while endoderm progenitors display a partial EMT signature [Citation28].
Figure 2. Examples of non-apical mitosis occurrence in development. (a) Representation of a transverse section from a gastrulating embryonic day (E7) mouse embryo. At the primitive streak (right square), cells undergo epithelial to mesenchymal transition and migrate away from the epibast. Mitosis can be found all along the apical-basal axis. (b) Representation of a neural tube transverse section from an HH13+ (-48h) chick embryo. Neural crest cells delaminate from the neural tube roof plate, and mitosis can be found all along the apical-basal axis. (c) Representation of interkinetic nuclear migration. In pseudostratified epithelia, nuclei are classically translocated during G2 and cells undergo mitosis apically
Epithelial-mesenchymal transition
EMT, a versatile and reversible process through which a static epithelial cell acquires a mesenchymal phenotype and becomes motile, was first described in embryo development, notably through the pioneering work of Elisabeth Hay in chick [Citation29]. It is also involved in pathological circumstances, such as fibrosis or metastasis from epithelium-derived carcinomas [Citation30].
EMT usually involves loss or change in junction repertoire, reorganization of the cytoskeleton, switch from apical-basal polarity to front-rear polarity, and remodeling of the extracellular matrix [Citation12,Citation31]. It is however a complex process with no universal step, mechanism, or set of markers; its strategy is context-dependant and can be multimodal even within a specific population of cells in a given epithelium [Citation9,Citation32,Citation33].
A concept coined as “go or grow” or “divide or conquer” states that cells must stop dividing in order to become motile and invasive [Citation34,Citation35]. A dichotomy between migration and proliferation was indeed found, for example, during the establishment of the uterine-vulvar connection in Caenorhabditis elegans, where G1 arrest is necessary for anchor cell invasion [Citation36]. Similarly, the transcription factor Ets1 attenuates breast cancer cell growth while promoting invasiveness [Citation37]. However, examining the relationship between migration and division in multiple contexts indicates that no “one-size fits all” rule can be applied [Citation38,Citation39]. Intravital imaging of inoculated human colon cancer cells showed that cells in S/G2/M phases were more motile and invasive than G1 cells [Citation40]. In the mouse embryo, while the EMT-triggering transcription factor Snail blocks the cell cycle in the neural epithelium or allantois at E8.5 [Citation41], we and others found an increased mitotic index in the Snail-enriched primitive streak at E7 [Citation42,Citation43].
Apart from gastrulation, neural crest delamination from the chick neuroepithelium is another well-studied developmental process involving EMT in a pseudostratified epithelium (). Interestingly, while Ets1-positive cranial neural crest can delaminate in any phase of the cell cycle [Citation44], trunk neural crest cells can only exit the neuroepithelium in S phase [Citation45], highlighting the specificity of EMT regulation.
Temporal and spatial regulation of mitosis
The spatial and temporal regulation of mitotic entry orchestrates the pattern of forces associated with morphogenetic events. During gastrulation in Drosophila, mitotic entry is counterproductive in nascent mesoderm cells as it reverses apical constriction by interfering with medio-apical localization of ROCK (Rho-associated protein kinase). On the other hand, mitosis in neighboring cells promotes invagination of contractile cells even when ectopically located [Citation46].
In pseudostratified epithelia, there is an additional dimension to the localization of cell division: along the apical-basal axis [Citation47]. Indeed, nuclei classically undergo InterKinetic Nuclear Migration (IKNM): they are localized at the basal side of the epithelium in S phase, then translocate toward the apical side during G2, and mitosis occurs at the most apical position [Citation48] (). First described in pig and chick neuroepithelium in 1935 [Citation49], IKNM has since been observed in many vertebrates in diverse neuroepithelia [Citation50,Citation51] as well as in other tissues such as liver, lung and pancreatic buds [Citation52]. It is an evolutionary conserved mechanism also found in invertebrates, notably in Nematostella ectoderm and Drosophila wing disc [Citation53]. Live imaging has confirmed the existence of such nuclear movements in the mouse epiblast [Citation54]. The movement of nuclei toward the apical side in G2/M, called PRAM (Pre-mitotic Rapid Apical Movement), is actively driven by cytoskeletal networks. PRAM can be mediated by microtubules and/or actomyosin, and the type of filaments involved appears to depend on the apical-basal length of the epithelium [Citation55]. Shorter pseudostratified epithelia, such as the zebrafish retina, rely mostly on actomyosin for IKNM [Citation56], while PRAM in longer pseudostratified epithelia like chick neuroepithelium is driven by both microtubules and actin [Citation57]. In Drosophila wing disc epithelium, the mechanisms that regulate nuclear movements depend on tissue architecture, notably cell density and apical curvature [Citation58]. Apical to basal movement in G1/S is described as passive and stochastic [Citation56]. Recent work using the zebrafish retina as model pseudostratified epithelium proposed a diffusive model, in which a gradient of concentration drives the average movement of particles (nuclei) from a high concentration area (apical side) to a low concentration area (basal side) [Citation59]. The mouse epiblast, a short pseudostratified epithelium, is particular in the way that cells undergoing mitotic rounding appear to completely detach from the basal pole, which could impact tissue reorganization [Citation42].
Non-apical mitosis, defined as occurring away from the apical border at a distance equal or superior to twice the nucleus size, has been observed in a series of pseudostratified epithelia (). As first reported by Snow in 1977 [Citation60], non-apical mitoses are frequent in the primitive streak throughout mouse gastrulation. Indeed, while very rare in the anterior and posterior epiblast, 40% of cell divisions at the streak are non-apical. In chick neuroepithelium, non-apical mitoses are present in approximatively the same proportion in pre-migratory cells undergoing EMT, and it has been suggested that ectopic mitoses might constitute an alternative mechanism for neural crest cells delamination [Citation57,Citation61].
Destabilization of the apical PAR complex assembly by PAR3 overexpression [Citation13] or downregulation [Citation61] is sufficient to trigger non-apical mitosis in chick neuropithelium. During mouse gastrulation, delaminating cells retain a connection to the apical compartment, and therefore polarity, until just before leaving the epiblast [Citation26]. It is thus not very likely that the appearance of non-apical mitosis is a consequence of apical compartment disorganization in that context. Disturbance of actomyosin dynamics may also impact the localization of mitosis. For example, inhibition of actin polymerization using latrunculin or cytochalasin B leads to an increased number of non-apical mitosis in the Drosophila wing disc [Citation53] or the chick neuroepithelium [Citation57].
Mitosis and cell differentiation
During mitosis, cell type specific programs are temporally abrogated: transcription is repressed, transcriptional activators and repressors dissociate from condensed chromatin and disperse in the cytoplasm [Citation62]. In addition, mitotic rounding is associated with nuclear membrane breakdown, cytoskeletal remodeling, and redistribution of organelles [Citation25,Citation63,Citation64]. In specific contexts, this labile state may provide an opportunity to transition between different states of gene expression [Citation64,Citation65] and/or favor cell shape changes.
The association between mitosis and cell differentiation is illustrated by asymmetrical division. In Drosophila neurogenesis, it involves segregation of cell fate determinants between daughter cells: division of neuroblasts generates a large cell that remains a neuroblast and a small one that differentiates [Citation66,Citation67]. During neurogenesis in mammalian embryos, cleavage orientation predicts the fate of daughter cells: progenitor cells displaying a horizontal division plane, so that only one of the daughter cells maintains contact with the niche, generate one cell that remains a progenitor and one cell that loses attachment and migrates basally to differentiate [Citation68,Citation69].
Mitosis, a springboard for EMT?
Among the many perturbations associated with mitosis that could impinge on EMT, we will focus on cell cycle, cell rounding, and the impact of adhesion remodeling on tissue rheology.
Cell cycle
During gastrulation, the mouse embryo undergoes massive cell proliferation: in the course of 24 h, the epiblast grows from around 660 to more than 16,000 cells [Citation70]. Mouse embryos with subnormal cell numbers due to cell loss or disruption of cell division fail to initiate gastrulation [Citation5]. In addition, the mitotic index is regionally regulated in the epiblast, such that mitoses are more frequent at the primitive streak [Citation42,Citation60]. Single-cell RNA analysis confirmed a higher proportion of cells in G2/M at the primitive streak [Citation43]. The identification of an asymmetry in epiblast proliferation raised several hypotheses. Snow proposed the existence of a “proliferative zone” where cells destined to ingress through the streak would be generated [Citation60]. MacAuley, who made similar observations in rat embryos, speculated that cells needed to go through an ultimate round of division before leaving the epiblast [Citation71]. During chick gastrulation, primitive streak progenitor cells undergo a bilateral counter-rotating (or “polonaise”) movement to reach the posterior side of the embryo [Citation72]. Formation and maturation of the avian primitive streak also involve convergence and extension movements [Citation73,Citation74]. However, in mouse epiblast, there is no polonaise movement, but rather a local recruitment of cells for ingression [Citation26]. Since cells are exiting the streak, the pool of dividing cells is constantly changing [Citation75], which could be interpreted as a “mitotic treadmill”. Apart from generating enough material for delamination through a higher frequency of cell division, cell cycle regulation could directly participate in the EMT process.
In addition, specifically in pseudostratified epithelia, the cell cycle involves nuclear movement along the apical-basal axis. Non-apical mitosis was observed in various pseudostratified epithelia upon alteration of the CyclinD1/CDK1 complex, the initiator of mitotic entry. In zebrafish retina, Cdk1 is required for PRAM and perturbation of IKNM leads to non-apical mitosis and loss of epithelial integrity [Citation76]. In chick neuroepithelium, overexpressing CyclinD1 triggers non-apical mitosis. In the mouse embryo, expression of CyclinD1 and D2 begins in the epiblast and nascent primitive streak, respectively, just a few hours before gastrulation, possibly explaining the contraction of the cell cycle at the streak, where cells have a short G1 [Citation77]. Interestingly, IKNM and the cell cycle can be uncoupled [Citation78]. An in silico 2D pseudostratified epithelium model based on data collected from chick neuroepithelium revealed that slowing IKNM was sufficient to promote non-apical mitosis [Citation79]. Therefore, it is possible that upon increase of the mitotic index, nuclear movements could no longer follow the pace of division, leading to desynchronization of the cell cycle and IKNM in some cells and consequently the appearance of non-apical mitosis.
Cell shape
Mitotic rounding during anaphase is necessary, for instance for spindle orientation and to enable microtubules to catch chromosomes, such that failure to change cell shape leads to mitotic delay or arrest [Citation63]. Mitotic rounding relies on actomyosin cytoskeleton remodeling, which is supported by small GTPases, notably RhoA. Entry into mitosis is controlled by the complex Cdk1/CyclinD1. Upon prophase, one of Cdk1 substrates, the RhoGEF Ect2, which plays an important role during cytokinesis [Citation16], leaves the nucleus. In the cytoplasm, Ect2 activates RhoA, which regulates myosin contractility via ROCK, allowing mitotic rounding [Citation14,Citation80]. Actin regulation during mitosis can also be mediated by other Rho GTPases, including Cdc42 and Rac1 [Citation81]. The implication of Rho GTPases is interesting since these proteins are also critical during EMT for the acquisition of a mesenchymal morphology, such as front-rear polarity, or formation of lamellipodia and filopodia [Citation12].
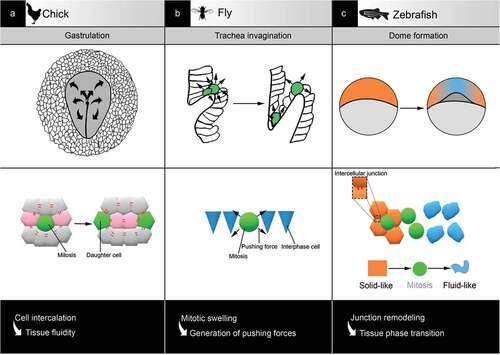
In addition to actin reorganization at the cortex, rounding requires mitotic swelling: an expansion of mitotic cell volume due to a change in osmolarity leading to increased hydrostatic pressure. Therefore, the dividing cell has the ability to exert forces on neighboring objects [Citation82,Citation83,Citation84], notably to escape epithelial confinement [Citation85] or proliferate in dense collagen-rich tissues [Citation86] (). In an epithelium, those forces might impact not only neighboring cells but also global tissue organization. For example, cell division drives cell rearrangements necessary for spatial patterning during chick gastrulation movements [Citation87] (). In zebrafish, apical mitotic rounding applies pulling forces on the luminal wall of the developing inner ear, allowing lumen expansion [Citation88]. Mechanical forces generated upon mitotic rounding help fasten up trachea invagination during fly development [Citation89] (). Interestingly, positive effects of mitotic entry on those processes were observed even if cytokinesis was blocked, emphasizing the impact of rounding itself.
Figure 3. Examples of tissue reorganisation associated with mitosis in development. (a) In chick, during gastrulation, mitosis is required for spatial patterning by favouring cell rearrangement and tissue fluidity. (b) In fly, during trachea invagination, mitosis accelerates the process via the generation of pushing forces against the environment. (C) In zebrafish, during dome formation, mitosis supports tissue phase transition by interfering with cell-cell adhesion
In the specific case of EMT, cells exiting an epithelium must break through the basement membrane, the last bastion before freedom. Cells classically produce matrix metalloproteases (MMP) that degrade matrix proteins [Citation12]. Indeed, basement membrane remodeling through Nodal-mediated MMP expression is one of the earliest steps in mouse gastrulation EMT [Citation26,Citation90]. We found that non-apical mitosis was associated with primitive streak morphogenesis even when the streak is ectopic [Citation42]. Since non-apical mitosis in the posterior epiblast was observed prior to the initiation of gastrulation, it is conceivable that basal mitotic cells pushing on a basement membrane might create an asymmetrical pattern of forces. This may synergize with MMP-driven destabilization and fasten up breakage, thereby facilitating the exit of not only daughter cells, but also non-dividing neighbors.
Rheology
Drawing parallels between biological tissues and materials may be fruitful to understand their behavior. Rheology studies the flow and deformation of a material, such as its solid and fluid characteristics [Citation91]. An abrupt change in macroscopic properties, such as from an ordered state of matter (solid-like) to a more disorganized state (liquid-like), is called a phase transition. It typically occurs when a “critical point”, defined in thermodynamics as the endpoint of phase equilibrium, is reached [Citation91,Citation92]. Such physical models have been used to describe epithelium dynamics [Citation91,Citation93]. Modifying individual cells’ parameters influences the global properties of a tissue, which then impacts morphogenesis [Citation87,Citation94,Citation95]. Recently, those concepts have been applied to embryonic tissues, which are often in a state close to criticality as they transit between robustness and adaptability [Citation91].
Embryonic tissues have inherent viscoelastic properties [Citation96,Citation97] whose spatial regulation is critical for embryo development. Modulation of cell density, notably through cell division, introduces dynamic reorganization in tissues with elastic properties that can lead to liquid-like behaviors [Citation94]. For example, in the zebrafish blastula, the loosening of cell-cell contacts upon cell division generates asymmetrical fluidization that facilitates dome formation [Citation98] (). Similarly, during chick gastrulation, cell division impacts the fluidity of the epiblast [Citation87,Citation95] (). Phase transitions can thus be triggered by relatively small changes in cell cohesion. As a general regulatory mechanism that modifies the physical properties of tissues, phase transition may benefit specific developmental processes [Citation99], including gastrulation EMT.
EMT also changes parameters defining the viscoelastic properties of an epithelium [Citation100]. Indeed, EMT can be assimilated to a “Jamming-Unjamming” phase transition [Citation101]. The “jamming” state, a term usually used for inert soft condensed matter, refers to a solid-like system portraying elastic behavior: it resists applied stress by deforming and returns to its original state once unload. “Unjamming” describes a fluid-like state [Citation102,Citation103]. As cell division has the capacity to influence tissue rheology, one could hypothesize that quantitative, spatial, and temporal regulation of mitosis may help reach a critical point for phase transition by favoring fluidization at sites of EMT.
Conclusion and perspectives
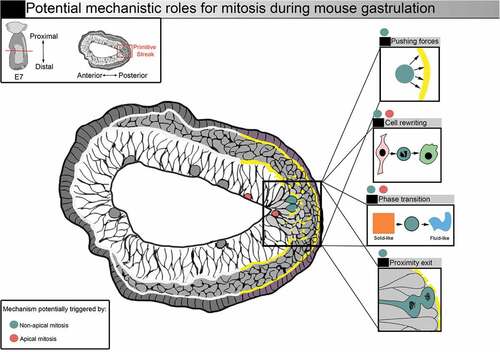
Gastrulation is a critical EMT event in development that leads to the generation of novel germ layers. In rodent embryos, mitotic index is increased at the primitive streak, which may play a role in maintaining cell packing in the entire epiblast through compensation of mechanical changes due to cell exit and asymmetrical apical surface shrinkage. However, the high apical contractility of streak cells may be deleterious to apical cell rounding and thus favor the appearance of non-apical mitosis. Moreover, as apical to basal IKNM movements follow a diffusion model which can be affected by apical crowding, the higher mitotic index at the primitive streak could desynchronize IKNM movement in this region compared to the rest of the epiblast [Citation59].
We found that the large majority of non-apical mitosis in the mouse embryo epithelial epiblast at the primitive streak gave rise to one or two mesenchymal mesoderm daughter cells, while apical mitosis resulted in epithelial epiblast daughter cells [Citation42]. Non-apical mitosis in the primitive streak might thus help the exit of nascent mesoderm cells from the epiblast through loss of cell-cell adhesion, modification of the spatial pattern of forces favoring basement membrane breakage, and/or increased epithelium fluidity (). Non-apical mitosis could be a cause or consequence of epithelial destabilization, and could, either way, be secondarily advantageous for EMT. To get further insight on the causality versus correlation relationship of time and space regulation of mitosis and EMT, further experiments could include live imaging of nuclear and cellular movements in different EMT models with modulation of the cell cycle, cytoskeleton, and extracellular remodeling through pharmacological and genetic approaches. Computational modeling based on specific parameters measured by live imaging is likely to be of great help to test hypotheses and integrate experimental results.
Figure 4. Potential mechanistic roles for mitosis during mouse gastrulation. Representation of a transverse section from a E7 mouse embryo. Squares on the right illustrate potential mechanistic roles for mitosis. Increased mitotic index (red and blue dots) could facilitate nascent mesoderm cell exit. Mitosis could benefit gastrulation by supporting shape and fate transition via mitotic cell rewriting. Forces generated by mitotic cells, as well as the remodelling of cell-cell adhesions, could also favour tissue phase transition. Entering mitosis in basal position (blue dots) could favor basement membrane breakage, and the generation of daughter cells close to the exit could facilitate delamination
In the last decade, technical breakthroughs have allowed the elaboration of embryo models from human and mouse embryonic stem cells, introducing novel strategies to study developmental processes. In contrast with the traditional “top-down” approach, which deconstructs embryos into modules, stem cells-based models work in reverse, building up complexity from simple blocks in a “bottom-up” fashion [Citation104]. The reproducibility of the models is compelling, highlighting the tremendous self-organization capacity of embryonic stem cells. They recapitulate numerous embryogenesis events, including symmetry breaking, EMT, and germ layer specification [Citation105,Citation106]. In addition, they allow a precise control of the physical parameters of the environment, which can unveil novel mechanosensitive signaling pathways. For instance, the appearance of “gastrulation-like” nodes in 2D culture of human embryonic stem cells on engineered patterned substrates led to the discovery of a cell adhesion tension-dependant spatial patterning of BMP4, which triggers Wnt signaling and mesoderm specification [Citation107]. However, the current 2D and 3D embryo models do not fully reproduce the geometry of native embryos. In particular, the pseudostratified epiblast organization is not present, precluding the study of the role of the position of mitosis in gastrulation EMT.
EMT highly depends on the cellular and tissular context, so that no rules can be strictly applied from one model to another. However, despite its apparent diversity, EMT is composed of simple modules that can differently combine and give rise to a robust and strongly adapted process. Numerous studies have highlighted the role of EMT and its reversion program (mesenchymal-epithelial transition or MET) in carcinoma progression and metastasis [Citation108,Citation109]. Although EMT is an attractive target for cancer treatment, its plasticity complexifies the choice of molecules and time window. Understanding the relationship between EMT and the temporal and spatial regulation of mitosis in relatively simple 3D physiological models such as gastrulation might help design experiments addressing the progression versus invasion paradigm in cancer models.
Disclosure statement
The authors declare that they have no financial or non-financial competing interests.
Additional information
Funding
References
- Cao X, Pfaff SL, Gage FH. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 2008;22(23):3320.
- Santamaría D, Barrière C, Cerqueira A, et al. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448(7155):811–815.
- Eisenhoffer GT, Rosenblatt J. Bringing balance by force: live cell extrusion controls epithelial cell numbers. Trends Cell Biol. 2013;23(4):185–192.
- Gudipaty SA, Rosenblatt J. Epithelial cell extrusion: pathways and pathologies. Semin Cell Dev Biol. 2017;67:132–140.
- Tam PPL, Behringer RR. Mouse gastrulation : the formation of a mammalian body plan. Mech Dev. 1997;68(1–2):3–25.
- Schneider I, Ellenberg J. Mysteries in embryonic development: how can errors arise so frequently at the beginning of mammalian life? PLoS Biol. 2019;17(3):e3000173.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674.
- Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120(11):1351–1383.
- Katsuno Y, Derynck R. Epithelial plasticity, epithelial-mesenchymal transition, and the TGF-beta family. Dev Cell. 2021;56(6):726–746.
- Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol. 2014;15(4):225–242.
- Matter K, Balda MS. SnapShot: epithelial tight junctions. Cell. 2014;157(4):992–992.e1.
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196.
- Afonso C, Henrique D. PAR3 acts as a molecular organizer to define the apical domain of chick neuroepithelial cells. J Cell Sci. 2006;119(20):4293–4304.
- Guillot C, Lecuit T. Adhesion disengagement uncouples intrinsic and extrinsic forces to drive cytokinesis in epithelial tissues. Dev Cell. 2013;24(3):227–241.
- Aguilar-Aragon M, Bonello TT, Bell GP, et al. Adherens junction remodelling during mitotic rounding of pseudostratified epithelial cells. EMBO Rep. 2020;21(4):e49700.
- Dix CL, Matthews HK, Uroz M, et al. The role of mitotic cell-substrate adhesion re-modeling in animal cell division. Dev Cell. 2018;45(1):132–145.e3.
- Ragkousi K, Marr K, Mckinney S, et al. Cell-cycle-coupled oscillations in apical polarity and intercellular contact maintain order in embryonic epithelia. Curr Biol. 2017;27(9):1381–1386.
- Bergstralh D, Lovegrove H, St Johnston D. Discs large links spindle orientation to apical-basal polarity in Drosophila epithelia. Curr Biol. 2013;23(17):1707–1712.
- Sawyer JM, Harrell JR, Shemer G, et al. Apical constriction: a cell shape change that can drive morphogenesis. Dev Biol. 2010;341(1):5–19.
- Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13(1):119–146.
- Thorpe SD, Lee DA. Dynamic regulation of nuclear architecture and mechanics—a rheostatic role for the nucleus in tailoring cellular mechanosensitivity. Nucleus. 2017;8(3):287–300.
- Zheng Y, Pan D. The hippo signaling pathway in development and disease. Dev Cell. 2019;50(3):264–282.
- Gudipaty SA, Lindblom J, Loftus PD, et al. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature. 2017;543(7643):118–121.
- Eisenhoffer GT, Loftus PD, Yoshigi M, et al. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484(7395):546–549.
- Devany J, Sussman DM, Yamamoto T, et al. Cell cycle-dependent active stress drives epithelia remodeling. PNAS. 2021;118(10):e197853118.
- Williams M, Burdsal C, Periasamy A, et al. Mouse primitive streak forms in situ by initiation of epithelial to mesenchymal transition without migration of a cell population. Dev Dyn. 2012;241(2):270–283.
- Ramkumar N, Omelchenko T, Silva-Gagliardi NF, et al. Crumbs2 promotes cell ingression during the epithelial-to-mesenchymal transition at gastrulation. Nat Cell Biol. 2016;18(12):1281–1291.
- Probst S, Tosic J, Schwan C, et al. Spatiotemporal sequence of mesoderm and endoderm lineage segregation during mouse gastrulation. Development. 2021;148(1): dev193789. 10.1242/dev.193789.
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat. 1995;154(1):8–20.
- Thiery JP, Acloque H, Huang RYJ, et al. Epithelial-mesenchymal transition in development and disease. Cell. 2009;139(5):871–890.
- Nieto MA, Huang RY, Jackson RA, et al. Review EMT. Cell. 2016;166(1):21–45.
- Yang J, Antin P, Berx G, et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21(6):341–352.
- Campbell K, Casanova J. A common framework for EMT and collective cell migration. Development. 2016;143(23):4291–4300.
- Giese A, Loo MA, Tra N, et al. Dichotomy of astrocytoma migration and proliferation. Int J Cancer. 1996;67(2):275–282.
- Kohrman AQ, Matus DQ. Divide or conquer: cell cycle regulation of invasive behavior. Trends Cell Biol. 2017;27(1):12–25.
- Matus DQ, Lohmer LL, Kelley LC, et al. Invasive cell fate requires G1 cell-cycle arrest and histone deacetylase-mediated changes in gene expression. Dev Cell. 2015;35(2):162–174.
- Furlan A, Vercamer C, Bouali F, et al. Ets-1 controls breast cancer cell balance between invasion and growth. Int J Cancer. 2014;135(10):2317–2328.
- Liuq Y, Sánchez-Tilló E, Lu X, et al. The ZEB1 transcription factor acts in a negative feedback loop with miR200 downstream of ras and Rb1 to regulate Bmi1 expression. J Biol Chem. 2014;289(7):4116–4125.
- Lee J, Choi J-H, Joo C-K. TGF-beta1 regulates cell fate during epithelial-mesenchymal transition by upregulating survivin. Cell Death Dis. 2013;4(7):e714.
- Kagawa Y, Matsumoto S, Kamioka Y, et al. Cell cycle-dependent Rho GTPase activity dynamically regulates cancer cell motility and invasion in vivo. PLoS ONE. 2013;8(12):e83629.
- Vega S, Morales AV, Ocaña OH, et al. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18(10):1131–1143.
- Mathiah N, Despin-guitard E, Stower M, et al. Asymmetry in the frequency and position of mitosis in the mouse embryo epiblast at gastrulation. EMBO Rep. 2020;21(11):e50944.
- Mohammed H, Hernando-Herraez I, Savino A, et al. Single-cell landscape of transcriptional heterogeneity and cell fate decisions during mouse early gastrulation. Cell Rep. 2017;20(5):1215–1228.
- Théveneau E, Duband JL, Altabef M. Ets-1 confers cranial features on neural crest delamination. PLoS ONE. 2007;2(11):e1142.
- Burstyn-Cohen T, Kalcheim C. Association between the cell cycle and neural crest delamination through specific regulation of G1/S transition. Dev Cell. 2002;3(3):383–395.
- Ko CS, Kalakuntla P, Adam C,M. Apical constriction reversal upon mitotic entry underlies different morphogenetic outcomes of cell division. Mol Biol Cell. 2020;31(16):1663–1674.
- Salomoni P, Calegari F. Cell cycle control of mammalian neural stem cells : putting a speed limit on G1. Trends Cell Biol. 2010;20(5):233–243.
- Strzyz PJ, Matejcic M, and Norden C Heterogeneity, Cell Biology and Tissue Mechanics of Pseudostratified Epithelia: Coordination of Cell Divisions and Growth in Tightly Packed Tissues . Int. rev. cell mol. biol. 2016;325:. 89–118.
- Sauer FC. Mitosis in the neural tube. J Comp Neurol. 1935;62(2):377–405.
- Spear PC, Erickson CA. Interkinetic nuclear migration: a mysterious process in search of a function. Development Growth and Differentiation. 2012;54(3):306–316
- Norden C. Pseudostratified epithelia – cell biology, diversity and roles in organ formation at a glance. J Cell Sci. 2017;130(11):1859–1863.
- Bort R, Signore M, Tremblay K, et al. Hex homeobox gene controls the transition of the endoderm to a pseudostratified, cell emergent epithelium for liver bud development. Dev Biol. 2006;290(1):44–56.
- Meyer EJ, Ikmi A, Gibson MC. Interkinetic Nuclear Migration Is a Broadly Conserved Feature of Cell Division in Pseudostratified Epithelia. Curr Biol. 2011;21(6):485–491.
- Ichikawa T, Nakazato K, Keller PJ, et al. Live imaging of whole mouse embryos during gastrulation: migration analyses of epiblast and mesodermal cells. PLoS ONE. 2013;8(7):64506.
- Lee HO, Norden C. Mechanisms controlling arrangements and movements of nuclei in pseudostratified epithelia. Trends Cell Biol. 2013;23(3):141–150.
- Norden C, Young S, Link BA, et al. Actomyosin Is the main driver of interkinetic nuclear migration in the retina. Cell. 2009;138(6):1195–1208.
- Spear PC, Erickson CA. Apical movement during interkinetic nuclear migration is a two-step process. Dev Biol. 2012;370(1):33–41.
- Kirkland NJ, Yuen AC, Tozluoglu M, et al. Tissue mechanics regulate mitotic nuclear dynamics during epithelial development. Curr Biol. 2020;30(13):2419–2432.e4.
- Azizi A, Herrmann A, Wan Y, et al. Nuclear crowding and nonlinear diffusion during interkinetic nuclear migration in the zebrafish retina. ELife. 2020;9:e58635.
- Snow MHL. Gastrulation in the mouse: growth and regionalization of the epiblast. Development. 1977;42(1):293–303.
- Andrieu C, Montigny A, Bibonne A, et al. MMP14 is required for delamination of chick neural crest cells independently of its catalytic activity. Development. 2020;147(7): dev183954. 10.1242/dev.183954.
- Egli D, Birkhoff G, Eggan K. Mediators of reprogramming: transcription factors and transitions through mitosis. Nat Rev Mol Cell Biol. 2008;9(7):505–516.
- Lancaster O, LeBerre M, Dimitracopoulos A, et al. Mitotic rounding alters cell geometry to ensure efficient bipolar spindle formation. Dev Cell. 2013;25(3):270–283.
- Champion L, Linder MI, Kutay U. Cellular reorganization during mitotic entry. Trends Cell Biol. 2017;27(1):26–41.
- Soufi A, Dalton S. Cycling through developmental decisions: how cell cycle dynamics control pluripotency, differentiation and reprogramming. Development. 2016;143(23):4301–4311.
- Bowman SK, Neumüller RA, Novatchkova M, et al. The Drosophila NuMA homolog mud regulates spindle orientation in asymmetric cell division. Dev Cell. 2006;10(6):731–742.
- Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132(4):583–597.
- Gómez-López S, Lerner RG, Petritsch C. Asymmetric cell division of stem and progenitor cells during homeostasis and cancer. Cell Mol Life Sci. 2014;71(4):575–597.
- Chenn A, McConnell SK. Cleavage orientation and the assymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82(4):631–641.
- Fossat N, Jones V, Garcia-Garcia MJ, et al. Modulation of WNT signaling activity is key to the formation of the embryonic head. Cell Cycle. 2012;11(1):26–32.
- MacAuley A, Werb Z, Mirkes PE. Characterization of the unusually rapid cell cycles during rat gastrulation. Development. 1993;117(3):873–883.
- Gräper L. Die Primitiventwicklung des Hühnchens nach stereokinematographischen Untersuchungen, kontrolliert durch vitale Farbmarkierung und verglichen mit der Entwicklung anderer Wirbeltiere. Wilhelm Roux Arch Entwickl Mech Org. 1929;116(1):382–429
- Chuai M, Weijer CJ. The mechanisms underlying primitive streak formation in the chick embryo. Curr Top Dev Biol. 2008;81:135–156.
- Chuai M, Zeng W, Yang X, et al. Cell movement during chick primitive streak formation. Dev Biol. 2006;296(1):137–149.
- O’Farrell PH, Stumpff J, Tin ST. Embryonic cleavage cycles: how is a mouse like a fly? Curr Biol. 2004;14(1):R35–R45.
- Strzyz PJ, Lee HO, Leung LC, et al. Interkinetic nuclear migration is centrosome independent and ensures apical cell division to maintain tissue integrity. Dev Cell. 2015;32(2):203–219.
- Wianny F, Real FX, Mummery CL, et al. G1‐phase regulators, cyclin D1, cyclin D2, and cyclin D3: up‐regulation at gastrulation and dynamic expression during neurulation. Dev Dyn. 1998;212(1):49–62.
- Kosodo Y, Suetsugu T, Suda M, et al. Regulation of interkinetic nuclear migration by cell cycle-coupled active and passive mechanisms in the developing brain. EMBO J. 2011;30(9):1690–1704.
- Ferreira A, Despin-Guitard M, Duarte F, et al. Interkinetic nuclear movements promote apical expansion in pseudostratified epithelia at the expense of apicobasal elongation. PLoS Comput Biol. 2019;15(12):e1007171.
- Matthews HK, Delabre U, Rohn JL, et al. Changes in Ect2 localization couple actomyosin-dependent cell shape changes to mitotic progression. Dev Cell. 2012;23(2):371–383.
- Mitsushima M, Toyoshima F, Nishida E. Dual role of Cdc42 in spindle orientation control of adherent cells. Mol Cell Biol. 2009;29(10):2816–2827.
- Stewart MP, Helenius J, Toyoda Y, et al. Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature. 2011;469(7329):226–231.
- Zlotek-Zlotkiewicz E, Monnier S, Cappello G, et al. Optical volume and mass measurements show that mammalian cells swell during mitosis. J Cell Biol. 2015;211(4):765–774.
- Son S, Kang JH, Oh S, et al. Resonant microchannel volume and mass measurements show that suspended cells swell during mitosis. J Cell Biol. 2015;211(4):757–763.
- Sorce B, Escobedo C, Toyoda Y, et al. Mitotic cells contract actomyosin cortex and generate pressure to round against or escape epithelial confinement. Nat Commun. 2015;6(8872). DOI:10.1038/ncomms9872.
- Nam S, Lin Y-H, Kim T, et al. Cellular pushing forces during mitosis drive mitotic elongation in collagen gels. Adv Sci. 2021;8(4):2000403.
- Firmino J, Rocancourt D, Saadaoui M, et al. Cell division drives epithelial cell rearrangements during gastrulation in chick. Dev Cell. 2016;36(3):249–261.
- Hoijman E, Rubbini D, Colombelli J, et al. Mitotic cell rounding and epithelial thinning regulate lumen growth and shape. Nat Commun. 2015;6(7355). DOI:10.1038/ncomms8355
- Kondo T, Hayashi S. Mitotic cell rounding accelerates epithelial invagination. Nature. 2013;494(7435):125–129.
- Kyprianou C, Christodoulou N, Hamilton RS, et al. Basement membrane remodelling regulates mouse embryogenesis. Nature. 2020;582(7811):253–258.
- Petridou NI, Heisenberg C-P. Tissue rheology in embryonic organization. EMBO J. 2019;38(20):e102497.
- La Porta CAM, Zapperi S. Phase transitions in cell migration. Nat Rev Phys. 2020;2(10):516–517.
- Barton DL, Henkes S, Weijer CJ, et al. Active vertex model for cell-resolution description of epithelial tissue mechanics. PLoS Comput Biol. 2017;13(6):e1005569.
- Ranft J, Basan M, Elgeti J, et al. Fluidization of tissues by cell division and apoptosis. PNAS. 2010;107(49):20863–20868.
- Saadaoui M, Rocancourt D, Roussel J, et al. A tensile ring drives tissue flows to shape the gastrulating amniote embryo. Science. 2020;367(6476):453–458.
- Forgacs G, Foty RA, Shafrir Y, et al. Viscoelastic properties of living embryonic tissues: a quantitative study. Biophysj. 1998;74(5):2227–2234.
- Schötz E-M, Lanio M, Talbot JA, et al. Glassy dynamics in three-dimensional embryonic tissues. Journal of the Royal Society. 2013;10(89):20130726.
- Petridou NI, Grigolon S, Salbreux G, et al. Fluidization-mediated tissue spreading by mitotic cell rounding and non-canonical Wnt signalling. Nat Cell Biol. 2019;21(2):169–178.
- Petridou NI, Corominas-Murtra B, Heisenberg C-P, et al. Rigidity percolation uncovers a structural basis for embryonic tissue phase transitions. Cell. 2021;184(7):1914–1928.e19.
- Barriga EH, Mayor R. Adjustable viscoelasticity allows for efficient collective cell migration. Semin Cell Dev Biol. 2019;93:55–68.
- Bi D, Yang X, Marchetti MC, et al. Motility-driven glass and jamming transitions in biological tissues. Phys Rev. 2016;6(2):21011.
- Sadati SM, Qazvini NT, Krishnan R, et al. Collective migration and cell jamming. Differentiation. 2013;86(3):121–125.
- Park J-A, Hun Kim J, Bi D, et al. Unjamming and cell shape in the asthmatic airway. Nat Mater. 2015;14(10):1040–1048.
- Shahbazi MN, Zernicka-Goetz M. Deconstructing and reconstructing the mouse and human early embryo. Nat Cell Biol. 2018;20(8):878–887.
- Heemskerk I. Full of potential: pluripotent stem cells for the systems biology of embryonic patterning. Dev Biol. 2020;460(1):86–98.
- Simunovic M, Metzger JJ, Etoc F, et al. A 3D model of a human epiblast reveals BMP4-driven symmetry breaking. Nat Cell Biol. 2019;21(7):900–910.
- Muncie JM, Ayad NME, Lakins JN, et al. Mechanical tension promotes formation of gastrulation-like nodes and patterns mesoderm specification in human embryonic stem cells. Dev Cell. 2020;55(6):679–694.e11.
- Tsai JH, Yang J. Epithelial – mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27(20):2192–2206.
- Pastushenko I, Brisebarre A, Sifrim A, et al. Identification of the tumour transition states occurring during EMT. Nature. 2018;556(7702):463–468.
